Controversial results have been found in literature for the association between insulin resistance and sustained virologic response to standard chronic hepatitis C treatment. This study aims to provide a systematic literature review with meta-analysis, in order to evaluate if insulin resistance interferes with sustained virologic response in patients infected by the HCV genotype 1 versus HCV genotypes 2 and 3, undergoing treatment with interferon and ribavirin or pegylated interferon and ribavarin.
MethodsSystematic search was performed on main electronic databases until May 2012. Primary outcome was sustained virologic response, defined as undetectable levels of HCV-RNA six months after the end of treatment. Meta-analytic measure was estimated using Dersimonian and Laird's method, using Stata software.
ResultsThirteen studies involving 2238 infected patients were included. There was a statistically significant association between insulin resistance and lower sustained virologic response rate, and this difference occurred in HCV genotype G1 (OR: 2.23; 95% CI: 1.59–3.13) and G2/G3 (OR: 4.45; 95% CI: 1.59–12.49). In addition, a difference was seen in the cut-offs used for defining insulin resistance by Homeostasis Model Assessment of Insulin Resistance. To minimize this limitation, sub-analysis that excluded the studies that did not use 2 as a cut-off value was performed and the results still demonstrated association between insulin resistance and sustained virologic response, for both genotypic groups.
ConclusionThis meta-analysis provides evidence that elevated Homeostasis Model Assessment of Insulin Resistance is associated with a lower sustained virologic response rate in patients with hepatitis C treated with interferon and ribavirin or pegylated interferon and ribavarin, regardless of their genotype.
Chronic infection by the hepatitis C (HCV) virus is considered to be a major public health issue all over the world.1 Many viral and host factors have been implicated in disease progression and/or response to antiviral treatment. Among those factors insulin resistance (IR) is noteworthy. Different studies have demonstrated an association between HCV infection and increase in IR prevalence.2–6 IR is the main pathophysiological mechanism of the metabolic syndrome, depending on the tissue sensitivity to insulin.2,5 In hepatitis C IR is related to the presence of steatosis, faster fibrosis progression and increased risk of progression to cirrhosis and hepatocellular carcinoma.4,6–8
Molecular studies have demonstrated the capacity of hepatitis C virus to promote IR by interfering with intracellular insulin signaling, either by the virus itself or via an increase in the tumor necrosis factor (TNF-α) production. This cytokine or viral core proteins can alter the residues of serine from the insulin substrate 1 and 2 receptors, as well as the expression of the cytokine suppressor substrate (SOCS). These changes can block the transactivation of glucose transporters (GLUT-4) in the cells, suppressing glucose uptake, leading to a hyperinsulinism state.5,9–11 On the other hand, increasing SOCS-3 expression could reduce the response to treatment with interferon and ribavirin (IFN+RBV).11,12 In fact, many studies have demonstrated that the presence of IR, identified by the HOMA-IR index (Homeostasis Model Assessment of Insulin Resistance)13,14 is associated with lower rates of sustained virologic response (SVR) to treatment with pegylated interferon and ribavarin (PEG-IFN+RBV).15–19 Despite the evidence shown in clinical and molecular studies, controversial results have been found in clinical practice regarding the association between IR and response to the standard antiviral treatment for chronic hepatitis C.20–22
The recent introduction of protease inhibitors in the treatment of genotype 1 carriers increases significantly the treatment response rate, which does not seem to be influenced by IR.23,24 Nonetheless these drugs are not recommended to non-1 genotypes and, also, in many countries triple therapy will not be available to all patients and PEG-IFN+RBV will remain the standard treatment for many subjects with chronic hepatitis C.
The aim of the present study was to provide a systematic literature review with meta-analysis, in order to evaluate if the IR interferes with SVR in patients infected by the HCV genotype 1 (G1) versus HCV genotypes 2 and 3 (G2/G3), undergoing treatment with IFN+RBV or PEG-IFN α2a or α2b+RBV.
MethodsElectronic search was performed on the MEDLINE database (via PUBMED), Latin America and Caribbean Literature in Health Sciences (LILACS), EMBASE, Cochrane Central Register of Controlled Trials and The Cochrane Library until May 2012. Electronic search was complemented by manual search from bibliographic references and abstracts from selected papers.
The searches were made using the words “genotype”, “hepatitis C” and “insulin resistance” via controlled vocabulary MeSH for the PUBMED database and adapted to the other databases, according to their specificities. Language or time limits were not used.
The following pre-defined inclusion criteria were: systematic reviews, clinical trials, prospective or retrospective observational studies, which reported the correlation between HOMA-IR and SVR, in subjects infected by the HCV (G1 or G2/3), that were treatment naive, with 18 years old or above, from any ethnic group. The exclusion criteria were: case reports; studies not carried out in humans; coinfection with human immunodeficiency virus (HIV), and those with subjects undergoing a therapy different from the ones defined on this study (PEG-IFN+RBV or IFN+RBV).
The main outcome was SVR, defined by undetectable levels of HCV-RNA six months after the end of the treatment.
Two reviewers performed the search on the database using the strategies previously defined and selected the trials to be included in the review. Initially it was agreed that in case a consensus was not achieved, a third reviewer would be consulted regarding eligibility, and he would be responsible for the final decision.
The Stata software (11.2 version; Stata Corp., College Station, TX, USA) was used for the statistical analysis. The outcomes were evaluated by dichotomous variables, for which an odds ratio (OR) and a 95% confidence interval (CI) were calculated. A p-value≤0.05 was considered statistically significant. Initially the heterogeneity among the studies was investigated with the Cochran's Q test and I2 Higgins and Thompson's statistics, with equivalent significances. Moderate heterogeneity was identified for all the meta-analysis (I2 near 50% and less than 75%). Therefore, a random effects model was considered and the meta-analytic measure (OR) was estimated using Dersimonian and Laird's method, based on the assumption that there is heterogeneity among the studies.
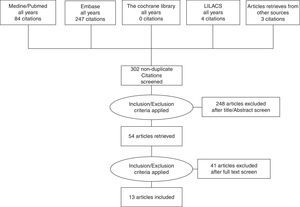
ResultsThe systematic literature review performed in June 2012 resulted in 84 bibliographic references from PUBMED, 4 bibliographic references from LILACS and 247 bibliographic references from EMBASE. After reading the titles and the abstracts, 281 references that did not meet the eligibility criteria or were duplicated, were excluded. Fifty-four papers were selected for more detailed analysis of their contents. In addition, papers from reference lists of the 54 selected papers and relevant paper reviews were added. At the end 13 studies that met the inclusion criteria were considered in the meta-analysis (Fig. 1).
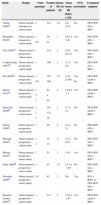
Characteristics of the included studiesThe characteristics of the included studies are described in Table 1. All the studies included are complete articles published in journals between 2005 and 2012. Most studies are prospective, observational, with only one being retrospective. All included subjects were treated with PEG-IFN α2a or α2b+RBV, with duration depending on the viral genotype. For IR definition, the HOMA-IR index>2 was considered, except in two studies that defined it as HOMA-IR>2.5,19,25 one that considered HOMA-IR>2.726 and another one that considered HOMA-IR>3.21 HOMA-IR and SVR showed statistically significant association in eight studies and, according to the genotypic classification of HCV, seven studies demonstrated positive association for the genotype 1 group and three studies for the genotypes 2 and 3. The other five studies did not find a significant association between HOMA-IR and SVR.
Studies included in the meta-analysis.
| Study | Design | Viral genotype | Number of patients | Homa-IR cut off | Mean homa-IR (index±SD) | SVR association | Treatment regimen |
|---|---|---|---|---|---|---|---|
| Akuta 200925 | Observational retrospective cohort study | 1 | 123 | 2.5 | 2.6 (2.6) | No | PEG-IFN α2b+RBV |
| Bortoletto 201021 | Observational prospective cohort study | 1 | 36 | 3 | 2.65±2.01 | Yes | PEG-IFN α2b+RBV |
| 2/3 | 21 | ||||||
| Chu 200818 | Observational prospective cohort study | 1 | 133 | 2 | 2.93 (0.14) | Yes | PEG-IFN α2b+RBV |
| Conjeevaram 200729 | Observational prospective cohort study | 1 | 399 | 2 | 3.5±5.0 | Yes | PEG-IFN α2a+RBV |
| Dai 200919 | Observational prospective cohort study | 1 | 150 | 2.5 | 2.24 (2.46) | Yes | PEG-IFN α2a/b+RBV |
| 2/3 | 180 | No | |||||
| Eslam 201230 | Observational prospective cohort study | 1 | 64 | 2 | 3.02±2.1 | Yes | PEG-IFN α2a/b+RBV |
| 2 | 4 | ||||||
| 3 | 48 | ||||||
| Fattovich 201020 | Observational prospective cohort study | 1 | 181 | 2 | 2.7±2.5 | No | PEG-IFN α2a/b+RBV |
| 2 | 131 | ||||||
| 3 | 78 | ||||||
| Grasso 200931 | Observational prospective cohort study | 1 | 90 | 2 | 2.6±2.1 | No | PEG-IFN α2b+RBV |
| Miyaaki 200932 | Observational prospective cohort study | 1 | 39 | 2 | 1.9±0.87 | No | PEG-IFN α2b+RBV |
| 2 | 12 | ||||||
| Mizuta 201033 | Observational prospective cohort study | 1 | 51 | 2 | 1.92 | Yes | PEG-IFN α2b+RBV |
| Petta 200926 | Observational prospective cohort study | 1 | 83 | 2.7 | 2.73±1.69 | No | PEG-IFN α2a+RBV |
| Poustchi 200816 | Observational prospective cohort study | 2/3 | 82 | 2 | NA | Yes | IFN+RBV or PEG-IFN α2b+RBV |
| Romero-Gomez 200515 | Observational prospective cohort study | 1 | 113 | 2 | 3.01±2.67 | Yes | PEG-IFN α2a/b+RBV |
HOMA, homeostasis model of assessment; SVR, sustained virologic response; NA, non-available; PEG-IFN, peginterferon; RBV, ribavirin.
Data calculated using available information.
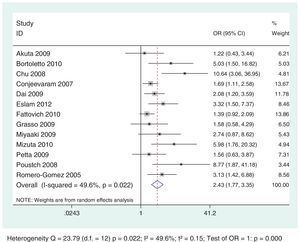
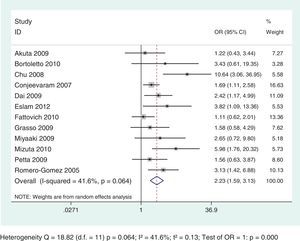
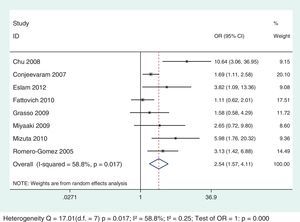
Thirteen selected studies, involving 2238 subjects, were included in the meta-analysis (Table 1). According to the analysis including all selected studies, the SVR rates were significantly lower in subjects with IR, when compared to those without IR, regardless of the genotype, using the random effects model by the Dersimonian and Laird's method (OR: 2.43; 95% CI: 1.77–3.35) (Fig. 2). Results remained unchanged even when studies that used for the definition of IR a HOMA-IR>2.5 or >2.7 or 3 were excluded from the analysis (OR: 2.54; 95% CI: 1.67–3.87) (Fig. 3).
To evaluate SVR rates in subjects with or without IR considering specific genotypes, 12 studies were included involving 2156 subjects with HCV G1, among which five studies also included subjects with other genotypes. Nevertheless, for the analysis, only the specific results for HCV G1 were considered. For the analysis of genotypes 2 and 3, six studies were included involving 552 subjects. Only results that reported data for genotypes 2 and 3 were added to the analysis.
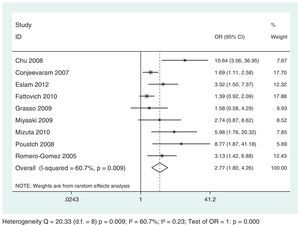
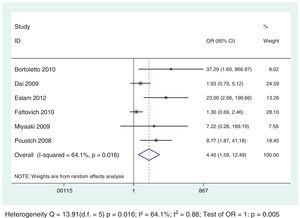
The analysis demonstrated a negative and statistically significant association between the HOMA-IR index and SVR rates regardless of HCV genotypes: G1 (OR: 2.23; 95% CI: 1.59–3.13) and G2/G3 (OR: 4.45; 95% CI: 1.59–12.49) (Figs. 4 and 5). For each genotypic group (HCV G1 and HCV G2/G3), the impact of IR on SVR rates was maintained, even after the exclusion of the studies with HOMA-IR index cut-off different from 2. (Data not shown – refer to supplementary data.)
This meta-analysis aimed to evaluate the impact of the IR on the response to antiviral treatment with IFN-RBV or PEG-IFN+RBV in subjects infected by HCV, taking into account the genotypic group G1 versus G2/G3. Thirteen studies were included involving 2238 infected subjects. The results demonstrated a statistically significant association between IR and lower SVR rates, regardless of the genotypic group evaluated, but was more evident on G2/G3: OR: 2.23; 95% CI: 1.59–3.13 and OR: 4.45; 95% CI: 1.59–12.49, respectively for HCV G1 and G2/G3.
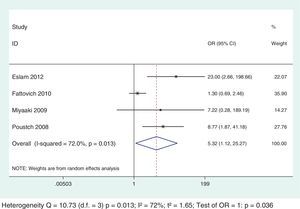
Among the selected studies for the meta-analysis, a difference in the cut-offs used for IR definition using the HOMA-IR index was noted. To minimize this limitation, sub-analysis that excluded the studies that did not use 2 as a cut-off value was performed and the results association between IR and SVR remained unchanged for both genotypic groups (Figs. 6 and 7).
The mechanism by which IR reduce the response to antiviral treatment in patients with HCV has not yet been completely elucidated. Nonetheless, it is known that IR presents different nuances according to the genotype. Therefore, while steatosis in genotype 1 hepatitis C is clearly associated with IR, in the genotype 3 carriers it seems to be caused basically by viral cytopathic effect.27,28 In this way, it is important to evaluate if IR interferes with the antiviral treatment response in patients with different genotypes. The studies available in the literature suggest an important role of IR in the pathogenesis of hepatitis C in response to the antiviral therapy related to the virus genotypes, specially among subjects with G1 HCV infection.15,18,29 In the study by Romero-Gomez and collaborators,15 it was demonstrated that IR strongly influenced SVR rates in patients infected by HCV G1, which varied from 60% in patients without IR to only 20% in patients with HOMA-IR>4. In this way, HOMA-IR index could be introduced as a predictive marker of SVR in patients infected by HCV G1. The multicentric study Virahep-C also evaluated how much IR interferes with SVR.29 This study was designed to evaluate the rates and predictors of SVR among Afro-American and Caucasian subjects infected by the HCV G1 under treatment with a combination of PEG-IFN+RBV. The HOMA-IR index value correlated significantly with SVR rates (highest SVR rates were associated with lowest HOMA-IR index value). In addition, the HOMA-IR value decreased during therapy between responders and non-responders, but this reduction was only statistically significant among those who achieved SVR. Chu and collaborators18 reported that IR played a decisive role on SVR in the treatment with PEG-IFN+RBV in Chinese subjects infected with HCV G1. Other studies support the data previously reported for G1.19,21,30–33
Currently, interest in the study of genotypes 2 and 3 has increased and two meta-analyses were conducted to demonstrate the relevance of IR in these cases. However, no comparisons versus genotype 1 were performed.34,35 In 2011, Eslam and collaborators published a meta-analysis aiming to evaluate the impact of IR on SVR in hepatitis C, but all genotypes were studied in this analysis (1, 2, 3 and 4), with no distinction among them.34 The study concluded that the increase in the HOMA-IR index was associated with lower therapeutic response in subjects infected by HCV.
A study published in 2008 by Poustchi and collaborators16 demonstrated that IR was an independent predictor of lower SVR in subjects infected by genotypes 2 or 3 (OR for no-response with HOMA-IR>2=6.5; 95% CI: 1.3–31.9; p=0.02). Another study in 62 subjects with different HCV genotypes (G1, G2 and G3) has demonstrated that SVR was reduced in patients with HOMA-IR>3, but this difference was not sustained when analyzed for each genotype individually, due to the limited number of subjects in each subgroup.21 However, Eslam30 with the purpose to investigate the effect of IR on SVR in 263 subjects with chronic hepatitis C, demonstrated that SVR rate was significantly reduced among patients with HOMA-IR≥2 for each genotype analyzed (G1, G3, and G4). This difference was maintained even when different cut-off values of HOMA-IR were used to define IR (>3 and >4). As such, the presence of IR, measured by the HOMA-IR index, presents prognostic value for standard therapy (PEG-IFN+RBV) also in patients with G2 or G3. This therapy tends to remain the standard treatment for these genotypes over the next years in Brazil.
Despite these results, the SVR variation according to HOMA-IR values remains a controversial issue, as some studies do not confirm that IR can play an important role in SVR.20,25,26,31 Fattovich et al.20 reported an association between HOMA-IR and rapid virologic response (RVR), but it was not predictive of SVR, in the entire cohort and also in HCV genotypes subgroups. In the same way, two other studies evaluating the response to therapy in subjects infected by G1 only reported association with RVR and IR, but did not find any significant association with SVR.26,31 Dai and Thompson suggested in their studies that viral eradication was associated with IR in patients infected by G1, but such association was not seen in patients infected by G2 or G3.19,22
This meta-analysis included the studies previously mentioned, initially assessing presence or absence of IR, regardless of the cut-off value used in HOMA-IR and, subsequently, including only those with cut-off value ≥2. On both it was clear that the presence of IR was associated with reduced SVR rates in patients infected by HCV G1 and G2/G3, and that HOMA-IR index can be used as a predictive factor for SVR in patients with G1, as well as on those with G2/G3. This an important point once there is no consensus regarding the HOMA-IR value limits that defines IR because values of 2; 2.5; 2.7 and 3 have been used. Besides, further studies to assess SVR in patients with G2 and G3 should be conducted to strengthen pre-existing data on its association with IR.
The discrepancy among the results in the selected studies must take into consideration that the interaction between IR and SVR also depends on other factors that need to be considered in the data interpretation. Besides the cut-off value used in the definition of IR, basal characteristics of the study cohort and the technical adequacy in collecting and measuring the serum insulin sample are of paramount importance. In this last case, different assays can present significant differences in determining serum insulin levels.36 On the other hand, both fasting glucose levels and serum insulin levels are potentially modifiable factors on a short period of time, through lifestyle changes (diet and exercise) as well as a result of medication.
Thus, patients with chronic hepatitis C that are candidates to antiviral therapy with PEG-IFN+RBV must be checked for IR before being started on treatment since HOMA-IR index presents a prognostic value for SVR in these patients, regardless of their genotypes. Although controversial, the modulation of insulin signaling and improvement in IR and serum glucose control should be attempted aiming at a better therapeutic response.37
Financial supportProfessional medical writing, statistician and editorial assistance were provided by ANOVA Consulting Group, Brazil and the study was funded by Bristol Myers Squibb/SA, Brazil.
Conflict of interestThe authors declare no conflicts of interest.
The authors would like to acknowledge the professional medical writing and the statistician for assistance in the preparation of this manuscript.