Ceftaroline, the active metabolite of the prodrug ceftaroline fosamil, is a cephalosporin with in vitro bactericidal activity against Gram-positive organisms, including methicillin-susceptible and -resistant Staphylococcus aureus, β-haemolytic and viridans group streptococci, and Streptococcus pneumoniae, as well as common Gram-negative organisms. In this study a total of 986 isolates collected in 2010 from patients in 15 medical centers in five Latin American countries from the Assessing Worldwide Antimicrobial Resistance Evaluation Program were identified as community-acquired respiratory tract or skin and soft tissue infection pathogens. Ceftaroline was the most potent agent tested against S. pneumoniae with a MIC90 value (0.12μg/mL) that was eight-fold lower than ceftriaxone, levofloxacin, and linezolid. Its spectrum of coverage (100.0% susceptible) was similar to tigecycline, linezolid, levofloxacin and vancomycin. Against Haemophilus influenzae and Moraxella catarrhalis, ceftaroline was the most active agent tested. The activity of ceftaroline against S. aureus (including MRSA) was similar to that of vancomycin and tetracycline (MIC90, 1μg/mL) and linezolid (MIC90, 2μg/mL). The β-haemolytic streptococci exhibited 100.0% susceptibility to ceftaroline. Ceftaroline activity against Escherichia coli, Klebsiella spp., and Enterobacter spp. was similar to that of ceftriaxone and ceftazidime. These parenteral cephalosporin agents have potent activity against non-extended-spectrum β-lactamase-phenotype strains, but are not active against extended-spectrum β-lactamase-phenotype strains. These results confirm the in vitro activity of ceftaroline against pathogens common in community-acquired respiratory tract and skin and soft tissue infection in Latin America, and suggest that ceftaroline fosamil could be an important therapeutic option for these infections.
Ceftaroline, the active form of the prodrug ceftaroline fosamil, is a new cephalosporin with in vitro activity against both Gram-positive (including methicillin-resistant Staphylococcus aureus [MRSA]) and common Gram-negative bacteria.1,2 It has been approved by the USA-FDA for use in community-acquired bacterial pneumonia (CABP) and in acute bacterial skin and skin structure infections (ABSSSI).3 It also has received marketing authorization in the European Union for community-acquired pneumonia (CAP) and complicated skin and soft tissue infections (cSSTI).4 The in vitro activity of ceftaroline against pathogens associated with CAP and cSSTI (as well as demonstrated clinical efficacy) make it an option for empiric monotherapy of these common infections.5–10
The emergence of drug-resistant bacteria causing CAP and cSSTI has increased the potential for inappropriate initial therapy in these infections.7–10 In CAP, multidrug resistance in Streptococcus pneumoniae has led to increased morbidity and mortality.11–13 In cSSTI, the emergence of multidrug-resistant MRSA has led to increased financial costs and increasing numbers of patients with unsatisfactory treatment outcomes due to decreasing therapy choices.7–9,14–16 The availability of ceftaroline, a β-lactam with activity against MRSA and S. pneumoniae, provides a much needed additional treatment option.1,2
With the commercial availability and use of ceftaroline fosamil, it is prudent to monitor activity of the drug against bacterial pathogens through surveillance studies to assess its continued activity and gather information on emerging resistance pathogens and mechanisms. The Assessing Worldwide Antimicrobial Resistance Evaluation (AWARE) Program is such a monitoring program.17–20 In this report, we present an evaluation of ceftaroline and comparator antimicrobial agents activity tested against 986 isolates from patients with community-acquired respiratory tract (CARTI) and skin and soft tissue (SSTI) infections as part of the AWARE Program in Latin American (LATAM) medical centers during 2010.
Materials and methodsOrganism collectionA total of 986 isolates from the AWARE Program identified as CARTI or SSTI pathogens by the infection type and/or specimen site recorded by the submitting laboratory were selected. Isolates were from patients in 15 medical centers in five LATAM countries (country, number of medical centers): Argentina (2), Brazil (6), Chile (2), Colombia (1) and Mexico (4). There were 312 CARTI isolates (S. pneumoniae, 172 [55.1%]; Haemophilus influenzae, 94 [30.1%]; and Moraxella catarrhalis, 46 [14.7%]) and 674 SSTI isolates (S. aureus, 370 [54.9%], β-haemolytic streptococci, 67 [9.9%]; Escherichia coli, (120 [17.8]; Klebsiella spp. 75 [11.1%]; and Enterobacter spp., 42, [6.2%]).
Susceptibility testingIsolates were tested for susceptibility to ceftaroline and comparator agents by reference broth microdilution methods.21 Clinical and Laboratory Standards Institute (CLSI) interpretations were based on criteria described in M100-S23.22 USA-FDA breakpoints were used for tigecycline in the absence of CLSI interpretations.23 Isolates were tested in cation-adjusted Mueller–Hinton broth (CA-MHB); supplemented with 2.5–5% lysed horse blood for streptococci.21Haemophilus spp. were tested in Haemophilus Test Medium.21 An extended spectrum β-lactamase (ESBL) phenotype was determined as per CLSI guidelines.22 Concurrent quality control (QC) testing was performed to assure proper test conditions and procedures. QC strains included: S. aureus ATCC 29213, Enterococcus faecalis ATCC 29212, S. pneumoniae ATCC 49619, H. influenzae ATCC 49247 and 49766, and E. coli ATCC 25922 and 35218. All QC results were within published CLSI ranges.22
ResultsThere were 172 S. pneumoniae isolates from CARTI, 48 (27.9%) of which exhibited penicillin-intermediate susceptibility (Pen-I; MIC, 0.12–1μg/mL) and 38 (22.1%) penicillin resistance (Pen-R, MIC, ≥2μg/mL). All strains were inhibited at a ceftaroline MIC of ≤0.5μg/mL with 100.0% of isolates categorized22 as susceptible (Tables 1 and 2). The MIC range for ceftaroline against penicillin-susceptible S. pneumoniae (ceftaroline MIC, ≤0.008–0.03μg/mL) was slightly lower than that of penicillin-intermediate (ceftaroline MIC, ≤0.008–0.12μg/mL) and penicillin-resistant (ceftaroline MIC, 0.12–0.5μg/mL) strains; see Tables 1 and 2. Ceftaroline was four- to eight-fold more active against penicillin-intermediate and eight-fold more active against penicillin-resistant strains than ceftriaxone (Table 2). Ceftaroline was also 16-fold more active than amoxicillin-clavulanate against the penicillin-intermediate strains and 32-fold more active against the penicillin-resistant isolates. All of the penicillin-intermediate and -resistant isolates were susceptible to ceftaroline, while only 73.7% of the penicillin-resistant isolates were susceptible to ceftriaxone (Table 2).
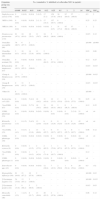
Cumulative % inhibited at each ceftaroline MIC when testing contemporary pathogens from Latin American CARTI and SSTI infections (2010).
| Organism group (no. tested) | No. (cumulative % inhibited) at ceftaroline MIC in (μg/ml): | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.008 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | ≥4 | MIC50 | MIC90 | |
| Staphylococcus aureus (370) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 17 (5.1) | 158 (47.8) | 39 (58.4) | 97 (84.6) | 57 (100.0) | – | 0.5 | 2 |
| MSSA (184) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.1) | 17 (10.3) | 157 (95.7) | 8 (100.0) | – | – | – | 0.25 | 0.25 |
| MRSA (186) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 31 (17.2) | 97 (69.4) | 57 (100.0) | – | 1 | 2 |
| Streptococcus pneumoniae (172) | 81 (47.1) | 24 (61.0) | 16 (70.3) | 11 (76.7) | 34 (96.5) | 5 (99.4) | 1 (100.0) | – | – | – | 0.015 | 0.12 |
| Penicillin-susceptible (86) | 78 (90.7) | 6 (97.7) | 2 (100.0) | – | – | – | – | – | – | – | ≤0.008 | ≤0.008 |
| Penicillin-intermediate (48) | 3 (6.3) | 18 (43.8) | 14 (72.9) | 11 (95.8) | 2 (100.0) | – | – | – | – | – | 0.03 | 0.06 |
| Penicillin-resistant (38) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 32 (84.2) | 5 (97.4) | 1 (100.0) | – | – | – | 0.12 | 0.25 |
| β-Haemolytic streptococci (67) | 29 (43.3) | 35 (95.5) | 3 (100.0) | – | – | – | -- | – | – | – | 0.015 | 0.015 |
| Group A Streptococcus (24) | 21 (87.5) | 3 (100.0) | – | – | – | – | – | – | – | – | ≤0.008 | 0.015 |
| Group B Streptococcus (34) | 0 (0.0) | 31 (91.2) | 3 (100.0) | – | – | – | – | – | – | – | 0.015 | 0.015 |
| Other (9) | 8 (88.9) | 1 (100.0) | – | – | – | – | – | – | – | – | ≤0.008 | – |
| Escherichia coli (120) | 0 (0.0) | 0 (0.0) | 5 (4.2) | 18 (19.2) | 31 (45.0) | 10 (53.3) | 2 (55.0) | 0 (55.0) | 3 (57.5) | 51 (100.0) | 0.25 | >4 |
| Non ESBL (67) | 0 (0.0) | 0 (0.0) | 5 (7.5) | 18 (34.3) | 31 (80.6) | 10 (95.5) | 2 (98.5) | 0 (98.5) | 1 (100.0) | – | 0.12 | 0.25 |
| ESBL phenotype (53) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.8) | 51 (100.0) | >4 | >4 |
| Klebsiella pneumoniae (67) | 1 (1.5) | 0 (1.5) | 2 (4.5) | 13 (23.9) | 6 (32.8) | 0 (32.8) | 0 (32.8) | 0 (32.8) | 0 (32.8) | 45 (100.0) | >4 | >4 |
| Non ESBL (23) | 1 (4.3) | 0 (4.3) | 2 (13.0) | 13 (69.6) | 6 (95.7) | 0 (95.7) | 0 (95.7) | 0 (95.7) | 0 (95.7) | 1 (100.0) | 0.06 | 0.12 |
| ESBL phenotype (44) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 44 (100.0) | >4 | >4 |
| Klebsiella oxytoca (8) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 2 (37.5) | 2 (62.5) | 1 (75.0) | 1 (87.5) | 0 (87.5) | 0 (87.5) | 1 (100.0) | 0.12 | – |
| Non ESBL (7) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 2 (42.9) | 2 (71.4) | 1 (85.7) | 1 (100.0) | – | – | – | 0.12 | – |
| ESBL phenotype (1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | >4 | – |
| Enterobacter spp. (42) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 (28.6) | 7 (45.2) | 2 (50.0) | 1 (52.4) | 0 (52.4) | 20 (100.0) | 0.5 | >32 |
| Haemophilus influenzae (94) | 61 (64.9) | 21 (87.2) | 10 (97.9) | 2 (100.0) | – | – | – | – | – | – | ≤0.008 | 0.03 |
| β-Lactamase negative (72) | 54 (75.0) | 13 (93.1) | 5 (100.0) | – | – | – | – | – | – | – | ≤0.008 | 0.015 |
| β-Lactamase positive (22) | 7 (31.8) | 8 (68.2) | 5 (90.9) | 2 (100.0) | – | – | – | – | – | – | 0.015 | 0.03 |
| Moraxella catarrhalis (46) | 1 (2.2) | 1 (4.3) | 8 (21.7) | 13 (50.0) | 20 (93.5) | 2 (97.8) | 1 (100.0) | – | – | – | 0.06 | 0.12 |
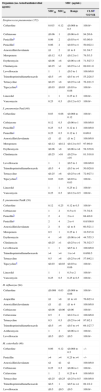
Activity of ceftaroline and comparator antimicrobial agents when tested against contemporary Latin American CARTI pathogens (2010).
| Organism (no. tested/antimicrobial agents) | MIC (μg/mL) | |||
|---|---|---|---|---|
| MIC50 | MIC90 | Range | CLSIa %S/%R | |
| Streptococcus pneumoniae (172) | ||||
| Ceftaroline | 0.015 | 0.12 | ≤0.008 to 0.5 | 100.0/– |
| Ceftriaxone | ≤0.06 | 1 | ≤0.06 to 8 | 94.2/0.6 |
| Penicillinb | 0.06 | 2 | ≤0.03 to 4 | 93.0/0.0 |
| Penicillinc | 0.06 | 2 | ≤0.03 to 4 | 50.0/22.1 |
| Amoxicillin/clavulanate | ≤1 | 2 | ≤1 to 8 | 91.3/4.7 |
| Meropenem | ≤0.12 | 0.5 | ≤0.12 to 1 | 79.5/3.5 |
| Erythromycin | ≤0.06 | >8 | ≤0.06 to >8 | 71.5/27.3 |
| Clindamycin | ≤0.25 | >1 | ≤0.25 to >1 | 89.0/11.0 |
| Levofloxacin | 1 | 1 | ≤0.5 to 1 | 100.0/0.0 |
| Trimethoprim/sulfamethoxazole | ≤0.5 | >4 | ≤0.5 to >4 | 55.2/28.5 |
| Tetracycline | ≤0.25 | >8 | ≤0.25 to >8 | 76.2/21.5 |
| Tigecyclined | ≤0.03 | ≤0.03 | ≤0.03 to 0.06 | 100.0/– |
| Linezolid | 1 | 1 | 0.25 to 2 | 100.0/– |
| Vancomycin | 0.25 | 0.5 | ≤0.12 to 0.5 | 100.0/– |
| S. pneumoniae PenI (48) | ||||
| Ceftaroline | 0.03 | 0.06 | ≤0.008 to 0.12 | 100.0/– |
| Ceftriaxone | 0.12 | 0.5 | ≤0.06 to 1 | 100.0/0.0 |
| Penicillinb | 0.25 | 0.5 | 0.12 to 1 | 100.0/0.0 |
| Penicillinc | 0.25 | 0.5 | 0.12 to 1 | 0.0/0.0 |
| Amoxicillin/clavulanate | ≤1 | ≤1 | ≤1 to 2 | 100.0/0.0 |
| Meropenem | ≤0.12 | ≤0.12 | ≤0.12 to 0.5 | 97.9/0.0 |
| Erythromycin | ≤0.06 | >8 | ≤0.06 to >8 | 58.3/39.6 |
| Clindamycin | ≤0.25 | >16 | ≤0.25 to >16 | 81.3/18.8 |
| Levofloxacin | 1 | 1 | ≤0.5 to 1 | 100.0/0.0 |
| Trimethoprim/sulfamethoxazole | ≤0.5 | 4 | ≤0.5 to >4 | 50.0/20.8 |
| Tetracycline | ≤0.25 | >8 | ≤0.25 to >8 | 72.9/27.1 |
| Tigecyclined | 0.03 | 0.03 | ≤0.03 to 0.03 | 100.0/– |
| Linezolid | 1 | 1 | 0.25 to 2 | 100.0/– |
| Vancomycin | 0.25 | 0.5 | ≤0.12 to 0.5 | 100.0/– |
| S. pneumoniae PenR (38) | ||||
| Ceftaroline | 0.12 | 0.25 | 0.12 to 0.5 | 100.0/– |
| Ceftriaxone | 1 | 2 | 0.5 to 8 | 73.7/2.6 |
| Penicillinb | 2 | 4 | 2 to 4 | 68.4/0.0 |
| Penicillinc | 2 | 4 | 2 to 4 | 0.0/100.0 |
| Amoxicillin/clavulanate | 2 | 8 | ≤1 to 8 | 60.5/21.1 |
| Meropenem | 0.5 | 1 | 0.25 to 1 | 10.5/15.8 |
| Erythromycin | 4 | >8 | ≤0.06 to >8 | 44.7/55.3 |
| Clindamycin | ≤0.25 | >1 | ≤0.25 to >1 | 76.3/23.7 |
| Levofloxacin | 1 | 1 | ≤0.5 to 1 | 100.0/0.0 |
| Trimethoprim/sulfamethoxazole | >4 | >4 | 1 to >4 | 0.0/89.5 |
| Tetracycline | 0.5 | >8 | ≤0.25 to >8 | 57.9/42.1 |
| Tigecyclined | ≤0.03 | ≤0.03 | ≤0.03 to 0.06 | 100.0/– |
| Linezolid | 1 | 1 | 0.5 to 2 | 100.0/– |
| Vancomycin | 0.25 | 0.5 | 0.25 to 0.5 | 100.0/– |
| H. influenzae (94) | ||||
| Ceftaroline | ≤0.008 | 0.03 | ≤0.008 to 0.06 | 100.0/– |
| Ampicillin | ≤1 | >8 | ≤1 to >8 | 76.6/23.4 |
| Amoxicillin/clavulanate | ≤1 | ≤1 | ≤1 to 4 | 100.0/0.0 |
| Ceftriaxone | ≤0.06 | ≤0.06 | ≤0.06 | 100.0/– |
| Cefuroxime | 0.5 | 2 | ≤0.12 to 4 | 100.0/0.0 |
| Tetracycline | 0.5 | 0.5 | ≤0.25 to >8 | 97.9/2.1 |
| Trimethoprim/sulfamethoxazole | ≤0.5 | >4 | ≤0.5 to >4 | 69.1/27.7 |
| Azithromycin | 1 | 2 | ≤0.06 to 4 | 100.0/– |
| Levofloxacin | ≤0.5 | ≤0.5 | ≤0.5 | 100.0/– |
| M. catarrhalis (46) | ||||
| Ceftaroline | 0.06 | 0.12 | ≤0.008 to 0.5 | –/– |
| Penicillin | >4 | >4 | 0.25 to >4 | –/– |
| Amoxicillin/clavulanate | ≤1 | ≤1 | ≤1 | 100.0/0.0 |
| Ceftriaxone | 0.25 | 0.5 | ≤0.06 to 1 | 100.0/– |
| Cefuroxime | 1 | 2 | 0.25 to 4 | 100.0/0.0 |
| Tetracycline | ≤0.25 | ≤0.25 | ≤0.25 to 0.5 | 100.0/0.0 |
| Trimethoprim/sulfamethoxazole | ≤0.5 | 1 | ≤0.5 to >4 | 89.1/2.2 |
| Levofloxacin | ≤0.5 | ≤0.5 | ≤0.5 | 100.0/– |
All 94 H. influenzae isolates were susceptible to ceftaroline with the highest MIC value at only 0.06μg/mL (Table 2). The MIC values for β-lactamase producing strains (MIC50, 0.015μg/mL and MIC90, 0.03μg/mL) were slightly higher than for β-lactamase-negative strains (MIC50≤0.008 and MIC90, 0.015μg/mL). Ceftaroline was also highly active against the 46 isolates of M. catarrhalis tested, with a MIC90 at 0.12μg/mL and a MIC range at ≤0.008–0.5μg/mL (Table 1).
There were a total of 370 S. aureus (50.3% MRSA) isolates from SSTI infections (Tables 1 and 3). The ceftaroline MIC50/90 for all S. aureus was at 0.5/2μg/mL; 84.6% susceptible22 and no resistant strains (≥4μg/mL; see Table 3). Ceftaroline activity against methicillin-susceptible S. aureus (MSSA) isolates (MIC50 and MIC90, 0.25μg/mL; 100% susceptible) was four- to eight-fold greater than noted for MRSA (MIC50/90, 1/2μg/mL; 69.4% susceptible) (Tables 1 and 3). The highest MIC results observed among MSSA and MRSA were 0.5 and 2μg/ml, respectively (Table 3). Ceftaroline was 16-fold more active than ceftriaxone when tested against MSSA. Most agents tested against MSSA from SSTI exhibited a high rate of susceptibility (>90%; see Table 3); exceptions were erythromycin and tetracycline (79.3 and 89.7%, respectively). Against all S. aureus (100.0% susceptible), linezolid (MIC50/90, 1/2μg/mL), vancomycin (MIC50/90, 1/1μg/mL), daptomycin (MIC50/90, 0.25/0.5μg/mL), and tigecycline (MIC50/90, 0.06/0.25μg/mL) were the most active agents. Only 10.2% of MRSA strains were susceptible to levofloxacin, 9.7% to erythromycin, and 13.4% to clindamycin (Table 3).
Activity of ceftaroline and comparator antimicrobial agents when tested against contemporary Latin American SSTI pathogens (2010).
| Organism (no. tested/antimicrobial agents) | MIC (μg/mL) | |||
|---|---|---|---|---|
| MIC50 | MIC90 | Range | CLSIa %S/%R | |
| Staphylococcus aureus (370) | ||||
| Ceftaroline | 0.5 | 2 | 0.06 to 2 | 84.6/0.0 |
| Ceftriaxone | 8 | >8 | 1 to >8 | 49.7/50.3 |
| Oxacillin | >2 | >2 | ≤0.25 to >2 | 49.7/50.3 |
| Meropenem | 1 | >8 | ≤0.12 to >8 | 49.7/50.3 |
| Erythromycin | >4 | >4 | ≤0.25 to >4 | 44.3/54.1 |
| Clindamycin | ≤0.25 | >2 | ≤0.25 to >2 | 54.6/45.1 |
| Levofloxacin | ≤0.5 | >4 | ≤0.5 to >4 | 52.7/45.9 |
| Trimethoprim/sulfamethoxazole | ≤0.5 | ≤0.5 | ≤0.5 to >4 | 97.0/3.0 |
| Tetracycline | ≤0.25 | 1 | ≤0.25 to >8 | 92.7/6.2 |
| Tigecyclineb | 0.06 | 0.25 | ≤0.03 to 0.5 | 100.0/– |
| Linezolid | 1 | 2 | 0.5 to 2 | 100.0/0.0 |
| Vancomycin | 1 | 1 | 0.5 to 2 | 100.0/0.0 |
| Daptomycin | 0.25 | 0.5 | 0.12 to 1 | 100.0/– |
| MRSA (186) | ||||
| Ceftaroline | 1 | 2 | 0.25 to 2 | 69.4/0.0 |
| Ceftriaxone | >8 | >8 | 8 to >8 | 0.0/100.0 |
| Oxacillin | >2 | >2 | >2 | 0.0/100.0 |
| Meropenem | >8 | >8 | 0.5 to >8 | 0.0/100.0 |
| Erythromycin | >4 | >4 | ≤0.25 to >4 | 9.7/89.8 |
| Clindamycin | >2 | >2 | ≤0.25 to >2 | 13.4/86.6 |
| Levofloxacin | >4 | >4 | ≤0.5 to >4 | 10.2/88.7 |
| Trimethoprim/sulfamethoxazole | ≤0.5 | ≤0.5 | ≤0.5 to >4 | 94.6/5.4 |
| Tetracycline | ≤0.25 | 1 | ≤0.25 to >8 | 95.7/4.3 |
| Tigecyclineb | 0.06 | 0.25 | ≤0.03 to 0.5 | 100.0/– |
| Linezolid | 1 | 1 | 0.5 to 2 | 100.0/0.0 |
| Vancomycin | 1 | 1 | 0.5 to 2 | 100.0/0.0 |
| Daptomycin | 0.25 | 0.5 | 0.25 to 1 | 100.0/– |
| MSSA (184) | ||||
| Ceftaroline | 0.25 | 0.25 | 0.06 to 0.5 | 100.0/0.0 |
| Ceftriaxone | 4 | 4 | 1 to 8 | 100.0/0.0 |
| Oxacillin | 0.5 | 0.5 | ≤0.25 to 1 | 100.0/0.0 |
| Meropenem | ≤0.12 | ≤0.12 | ≤0.12 to 0.25 | 100.0/0.0 |
| Erythromycin | ≤0.25 | >4 | ≤0.25 to >4 | 79.3/17.9 |
| Clindamycin | ≤0.25 | ≤0.25 | ≤0.25 to >2 | 96.2/3.3 |
| Levofloxacin | ≤0.5 | ≤0.5 | ≤0.5 to >4 | 95.7/2.7 |
| Trimethoprim/sulfamethoxazole | ≤0.5 | ≤0.5 | ≤0.5 to >4 | 99.5/0.5 |
| Tetracycline | ≤0.25 | 8 | ≤0.25 to >8 | 89.7/8.2 |
| Tigecyclineb | 0.06 | 0.25 | ≤0.03 to 0.5 | 100.0/– |
| Linezolid | 1 | 2 | 0.5 to 2 | 100.0/0.0 |
| Vancomycin | 1 | 1 | 0.5 to 2 | 100.0/0.0 |
| Daptomycin | 0.25 | 0.5 | 0.12 to 0.5 | 100.0/– |
| β-haemolytic streptococci (67) | ||||
| Ceftaroline | 0.015 | 0.015 | ≤0.008 to 0.03 | 100.0/– |
| Penicillin | ≤0.03 | 0.06 | ≤0.03 to 0.06 | 100.0/– |
| Ceftriaxone | ≤0.06 | 0.12 | ≤0.06 to 0.12 | 100.0/– |
| Meropenem | ≤0.12 | ≤0.12 | ≤0.12 | 100.0/– |
| Erythromycin | ≤0.25 | ≤0.25 | ≤0.25 to >4 | 94.0/6.0 |
| Clindamycin | ≤0.25 | ≤0.25 | ≤0.25 to >2 | 94.0/6.0 |
| Levofloxacin | ≤0.5 | 1 | ≤0.5 to 4 | 98.5/0.0 |
| Trimethoprim/sulfamethoxazole | ≤0.5 | ≤0.5 | ≤0.5 to >4 | –/– |
| Tetracycline | >8 | >8 | ≤0.25 to >8 | 37.3/62.7 |
| Tigecyclineb | ≤0.03 | ≤0.03 | ≤0.03 to 0.06 | 100.0/– |
| Linezolid | 1 | 1 | 0.5 to 1 | 100.0/– |
| Vancomycin | 0.5 | 0.5 | 0.25 to 0.5 | 100.0/– |
| Daptomycin | 0.12 | 0.25 | ≤0.06 to 0.25 | 100.0/– |
| E. coli (120) | ||||
| Ceftaroline | 0.25 | >32 | 0.03 to >32 | 55.0/45.0 |
| Ceftazidime | 0.25 | 32 | 0.06 to >32 | 67.5/26.7 |
| Ampicillin | >8 | >8 | 2 to >8 | 17.5/82.5 |
| Ampicillin/sulbactam | 16 | >32 | 1 to >32 | 25.8/40.0 |
| Piperacillin/tazobactam | 2 | 16 | 1 to >64 | 90.8/3.3 |
| Tetracycline | >8 | >8 | 0.5 to >8 | 32.5/67.5 |
| Tigecyclineb | 0.12 | 0.5 | 0.06 to 1 | 100.0/0.0 |
| Ceftriaxone | ≤0.06 | >8 | ≤0.06 to >8 | 57.5/42.5 |
| Gentamicin | ≤1 | >8 | ≤1 to >8 | 74.2/25.8 |
| Levofloxacin | 4 | >4 | ≤0.5 to >4 | 49.2/49.2 |
| Meropenem | ≤0.12 | ≤0.12 | ≤0.12 | 100.0/0.0 |
| E. coli non-ESBL (67) | ||||
| Ceftaroline | 0.12 | 0.25 | 0.03 to 2 | 98.5/1.5 |
| Ceftazidime | 0.12 | 0.25 | 0.06 to 0.5 | 100.0/0.0 |
| Ampicillin | >8 | >8 | 2 to >8 | 31.3/68.7 |
| Ampicillin/sulbactam | 16 | 32 | 1 to >32 | 41.8/19.4 |
| Piperacillin/tazobactam | 2 | 4 | 1 to 64 | 98.5/0.0 |
| Tetracycline | >8 | >8 | 0.5 to >8 | 43.3/56.7 |
| Tigecyclineb | 0.12 | 0.5 | 0.06 to 1 | 100.0/0.0 |
| Ceftriaxone | ≤0.06 | ≤0.06 | ≤0.06 to 0.12 | 100.0/0.0 |
| Gentamicin | ≤1 | >8 | ≤1 to >8 | 89.6/10.4 |
| Levofloxacin | ≤0.5 | >4 | ≤0.5 to >4 | 71.6/26.9 |
| Meropenem | ≤0.12 | ≤0.12 | ≤0.12 | 100.0/0.0 |
| K. pneumoniae (67) | ||||
| Ceftaroline | >32 | >32 | ≤0.008 to >32 | 32.8/67.2 |
| Ceftazidime | 16 | >32 | 0.03 to >32 | 46.3/52.2 |
| Ampicillin | >8 | >8 | ≤1 to >8 | 6.0/94.0 |
| Ampicillin/sulbactam | >32 | >32 | ≤0.25 to >32 | 32.8/64.2 |
| Piperacillin/tazobactam | 16 | >64 | ≤0.5 to >64 | 53.7/35.8 |
| Tetracycline | 2 | >8 | 0.5 to >8 | 74.6/20.9 |
| Tigecyclineb | 0.5 | 1 | 0.12 to 4 | 97.0/0.0 |
| Ceftriaxone | >8 | >8 | ≤0.06 to >8 | 34.3/65.7 |
| Gentamicin | ≤1 | >8 | ≤1 to >8 | 56.7/41.8 |
| Levofloxacin | 1 | >4 | ≤0.5 to >4 | 53.7/46.3 |
| Meropenem | ≤0.12 | 8 | ≤0.12 to >8 | 82.1/14.9 |
| K. pneumoniae non-ESBL (51) | ||||
| Ceftaroline | 0.06 | 0.12 | ≤0.008 to 4 | 95.7/4.3 |
| Ceftazidime | 0.12 | 0.25 | 0.03 to 1 | 100.0/0.0 |
| Ampicillin | >8 | >8 | ≤1 to >8 | 17.4/82.6 |
| Ampicillin/sulbactam | 4 | 8 | ≤0.25 to >32 | 95.7/4.3 |
| Piperacillin/tazobactam | 2 | 4 | ≤0.5 to >64 | 95.7/4.3 |
| Tetracycline | 1 | 8 | 0.5 to >8 | 87.0/4.3 |
| Tigecyclineb | 0.25 | 0.5 | 0.12 to 2 | 100.0/0.0 |
| Ceftriaxone | ≤0.06 | 0.12 | ≤0.06 to 0.5 | 100.0/0.0 |
| Gentamicin | ≤1 | ≤1 | ≤1 | 100.0/0.0 |
| Levofloxacin | ≤0.5 | >4 | ≤0.5 to >4 | 87.0/13.0 |
| Meropenem | ≤0.12 | ≤0.12 | ≤0.12 | 100.0/0.0 |
| K. oxytoca (8) | ||||
| Ceftaroline | 0.12 | – | 0.03 to 16 | 87.5/12.5 |
| Ceftazidime | 0.06 | – | 0.06 to 0.5 | 100.0/0.0 |
| Ampicillin | >8 | – | >8 | 0.0/100.0 |
| Ampicillin/sulbactam | 4 | – | 2 to >32 | 75.0/25.0 |
| Piperacillin/tazobactam | 1 | – | 1 to >64 | 87.5/12.5 |
| Tetracycline | 1 | – | 0.5 to 1 | 100.0/0.0 |
| Tigecyclineb | 0.12 | – | 0.12 to 0.5 | 100.0/0.0 |
| Ceftriaxone | ≤0.06 | – | ≤0.06 to 2 | 87.5/0.0 |
| Gentamicin | ≤1 | – | ≤1 | 100.0/0.0 |
| Levofloxacin | ≤0.5 | – | ≤0.5 to 1 | 100.0/0.0 |
| Meropenem | ≤0.12 | – | ≤0.12 | 100.0/0.0 |
| Enterobacter spp. (42) | ||||
| Ceftaroline | 0.5 | >32 | 0.12 to >32 | 50.0/47.6 |
| Ceftazidime | 1 | >32 | 0.12 to >32 | 64.3/33.3 |
| Ampicillin | >8 | >8 | 2 to >8 | 7.1/92.9 |
| Ampicillin/sulbactam | 32 | >32 | 4 to >32 | 21.4/57.1 |
| Piperacillin/tazobactam | 4 | >64 | 1 to >64 | 66.7/14.3 |
| Tetracycline | 2 | >8 | 1 to >8 | 76.2/16.7 |
| Tigecyclineb | 0.25 | 1 | 0.12 to 4 | 95.2/0.0 |
| Ceftriaxone | 1 | >8 | ≤0.06 to >8 | 52.4/45.2 |
| Gentamicin | ≤1 | >8 | ≤1 to >8 | 71.4/23.8 |
| Levofloxacin | ≤0.5 | >4 | ≤0.5 to >4 | 73.8/23.8 |
| Meropenem | ≤0.12 | ≤0.12 | ≤0.12 to 0.5 | 100.0/0.0 |
All 67 strains of β-haemolytic streptococci were susceptible to ceftaroline (MIC50/90, 0.015/0.015μg/mL; Table 3). Ceftaroline activity was slightly greater against the Group A serogroup (MIC50/90, ≤0.008/0.015μg/mL) than against Group B (MIC50/90, 0.015/0.015μg/mL). All Group A isolates and 91.2% of Group B isolates exhibited a MIC value of ≤0.015μg/mL and there were only 3 Group B isolates at the highest MIC value of 0.03μg/mL (Table 1). Tetracycline susceptibility for the Group A serotype strains was 62.5% (data not shown). Susceptibility was 95.8% for erythromycin, clindamycin, and levofloxacin (data not shown). For the Group B serotype, tetracycline susceptibility was 17.5%, while for erythromycin and clindamycin it was at 94.1% (data not shown).
A total of 44.2% of E. coli isolates were an ESBL-phenotype (Table 1). The ceftaroline MIC range for all E. coli was 0.03 to >32μg/mL with 55.0% of the isolates exhibiting susceptibility22 to ceftaroline (MIC50/90, 0.25/>32μg/mL) (Table 3). Ceftaroline was very potent against the non-ESBL-phenotype E. coli with a MIC50/90 at 0.12/0.25μg/mL; 98.5% susceptible at ≤0.5μg/mL.22 Decreased susceptibility was exhibited by other agents such as ampicillin-sulbactam (41.8%), gentamicin (89.6%), tetracycline (43.3%), and levofloxacin (71.6%) for the non-ESBL-phenotype strains (Table 3). Susceptibility rates were decreased even further for these agents against the ESBL-phenotype strains with susceptibility rates ranging from 5.7% (ampicillin-sulbactam) to 54.7% (gentamicin) (data not shown). None of the ESBL-phenotype strains tested were susceptible to ceftaroline.
For K. pneumoniae, ceftaroline was highly active against the non-ESBL-phenotype strains (MIC50/90, 0.06/0.12μg/mL; 95.7% susceptible) while susceptibility for levofloxacin (87.0%) and tetracycline (87.0%) was decreased (Table 3). All ESBL-phenotype strains were resistant to ceftaroline with concurrently low susceptibility to gentamicin (34.1%), levofloxacin (36.4%), and tetracycline (68.2%; data not shown). The highest ceftaroline MIC value for the seven non-ESBL phenotype Klebsiella oxytoca strains was 0.5μg/mL while the one ESBL-phenotype strain was ceftaroline resistant (Table 1).
Enterobacter spp. exhibited a ceftaroline MIC50 and MIC90 at 0.5 and >32μg/mL, respectively for 42 isolates (Tables 1 and 3). The ceftaroline susceptibility rate was similar at 50.0% to that of ceftriaxone (52.4%).
DiscussionCeftaroline demonstrated in vitro activity against the most common CARTI and SSTI pathogens isolated from patients in 15 LATAM medical centers. It was the most active agent tested against the CARTI pathogen S. pneumoniae with a MIC90 value that was eight-fold lower than ceftriaxone, levofloxacin, and linezolid. Ceftaroline's spectrum of coverage (at 100.0% susceptible) was similar to tigecycline, linezolid, and vancomycin. All of the above agents retained activity against penicillin-resistant strains (penicillin MIC, ≥2μg/mL). Ceftaroline was the most active agent tested against H. influenzae (100.0% susceptible, MIC90, 0.03μg/mL) and M. catarrhalis (MIC90, 0.12μg/mL; no interpretive criteria available). When tested against S. aureus, the activity of ceftaroline was similar to that of vancomycin and tetracycline (MIC90, 1μg/mL) and linezolid (MIC90, 2μg/mL). However, its coverage was reduced relative to these agents, as 15.4% of the staphylococci exhibited MIC values at 2μg/mL, which is the CLSI intermediate susceptibility category for ceftaroline.22 Against the β-haemolytic streptococci, the activity of ceftaroline was similar to daptomycin, linezolid, vancomycin, tigecycline, meropenem, penicillin and ceftriaxone; all providing complete (100.0%) coverage. The ceftaroline activity against E. coli, and Klebsiella spp. was similar to that of ceftriaxone and ceftazidime. These agents have potent activity against non-ESBL phenotype strains, but should be considered inactive against ESBL-phenotype Enterobacteriaceae.
Pfaller and colleagues20 conducted an evaluation of the activity of ceftaroline over a three year period in the USA (2008–2010). The ceftaroline MIC90 reported by Pfaller et al. for S. pneumoniae was 0.12μg/mL with 98.7% of MIC values ≤0.25μg/mL. Ceftaroline was shown to be 16-fold more active than ceftriaxone.20 Further the MIC90 values for H. influenzae and M. catarrhalis, respectively, were 0.015 and 0.12μg/mL. A total of 99.9% of H. influenzae were susceptible to ceftaroline; there are no interpretive criteria available for M. catarrhalis. In this 2010 LATAM AWARE Program report, the MIC90 for S. pneumoniae was also at 0.12μg/mL and susceptibility was at 100.0% using CLSI breakpoint criteria.22 The MIC90 for H. influenzae was lower in this study at 0.03μg/mL when compared to the Pfaller et al.20 study, with identical MIC90 values for M. catarrhalis (0.12μg/mL).
The activity of ceftaroline against SSTI pathogens from the 2010 LATAM surveillance presented here is comparable to that reported by Jones et al. from an international 2008 surveillance program conducted in the USA and Europe regions.5 In that study, the S. aureus MIC50/90 for ceftaroline was 0.5/1μg/mL, as compared to 0.5/2μg/mL in this 2010 LATAM sample. Jones et al. reported that there were regional differences for MRSA, with the MIC50/90 for ceftaroline in Europe at 1/2μg/mL while it was at 1/1μg/mL for the USA.5 The LATAM population of MRSA in this study, as with the European collection reported by Jones et al.,5 contains more strains of MRSA at the MIC value of 2μg/mL. This may be due to MRSA clonal differences between regions. A reduction in S. aureus with an MIC value at 2μg/mL in the USA has been shown by Farrell et al. to coincide with a shift toward the USA300 clone.18 Ceftaroline was also noted to be active against Enterobacteriaceae that are of the non-ESBL phenotype while it has limited activity against ESBL-producing organisms or strains overexpressing AmpC.1,5,17 This activity was consistent with other third generation cephalosporins such as ceftazidime and ceftriaxone.
In summary, ceftaroline demonstrated in vitro activity against a collection of contemporary Gram-positive and -negative pathogens from LATAM, associated with CARTI and SSTI infections. This suggests that ceftaroline fosamil merits further study in LATAM for these clinical indications.
Conflict of interestJMI Laboratories, Inc. has received research and educational grants in 2010–2012 from – Achaogen, Aires, American Proficiency Institute (API), Anacor, Astellas, AstraZeneca, bioMerieux, Cempra, Cerexa, Contrafect, Cubist, Dipexium, Enanta, Furiex, GlaxoSmithKline, Johnson & Johnson, LegoChem Biosciences Inc., Meiji Seika Kaisha, Nabriva, Novartis, Pfizer, PPD Therapeutics, Premier Research Group, Rempex, Rib-X Pharmaceuticals, Seachaid, Shionogi, The Medicines Co., Theravance, ThermoFisher and some other corporations. Some JMI employees are advisors/consultants for Astellas, Cubist, Pfizer, Cempra, Cerexa-Forest, and Theravance. In regards to speakers bureaus and stock options-none to declare.
The authors would like to thank the following investigators for kindly contributing strains to this study: Jorgelina Smayevsky (Laboratorio CEMIC, Buenos Aires, Argentina); Gabriella Tome and Jose M. Cassellas (Sanatorio Parque y Nino, Rosario, Sante Fe, Argentina); Julival Ribeiro (Hospital de Base, Brasilia, Brazil); Cassia Zoccoli (Laboratorio Medico Santa Luzia, Florianopolis, Brazil); Afonso Barth (Hospital de Clinicas, Porto Alegre, Brazil); Pedro Del Peloso (Samaritano Hospital, Rio de Janeiro, Brazil); Rosangela Cipriano, Hospital São Domingos, São Luis, Brazil); Ana C. Gales (Federal University of São Paulo, São Paulo, Brazil); Patricia Garcia (Catholic University Hospital, Santiago, Chile); Valeria Prado (Universidad de Chile, Santiago, Chile); Jaime Robledo (Laboratorio Medico de Referencia Ltda, Medellin, Colombia); Juan C. Tinoco (Hospital General de Durango, Durango, Mexico); Rayo Morfin (Instituto de Pathologia Infecciosa, Guadalajara, Mexico); Elvira Garza-Gonzalez (Universidad Autonoma de Nuevo Leon, Monterrey, Mexico); and Patricia Cornejo (Instituto Nacional de Cancerologia, Tlalpan, Mexico).
This study at JMI Laboratories was supported by an Educational/Research Grant from AstraZeneca, and JMI Laboratories received compensation fees for services in relation to preparing the manuscript, which was funded by AstraZeneca.