Several major epidemics of Zika fever, caused by the ZIKA virus (ZIKV), have emerged in Brazil since early 2015, eventually spreading to other countries on the South American continent. The present study describes the clinical manifestations and laboratory findings of patients with confirmed acute ZIKV infection during the first epidemic that occurred in Salvador, Brazil. All included patients were seen at the emergency room of a private tertiary hospital located in Salvador, Brazil from 2015 through 2017. Patients were considered eligible if signs of systemic viral febrile disease were present. All individuals were tested for ZIKV and Chikungunya infection using PCR, while rapid test was used to detect Dengue virus antibodies or, alternatively, the NS1 antigen. A diagnosis of acute ZIKV infection was confirmed in 78/434 (18%) individuals with systemic viral febrile illness. Positivity was mainly observed in blood, followed by saliva and urine. Coinfection with Chikungunya and/or Dengue virus was detected in 5% of the ZIKV-infected patients. The most frequent clinical findings were myalgia, arthralgia and low-grade fever. Laboratory analysis demonstrated normal levels of hematocrit, platelets and liver enzymes. In summary, in acute settings where molecular testing remains unavailable, clinicians face difficulties to confirm the diagnosis of ZIKV infection, as they rely only on clinical examinations and conventional laboratory tests.
Zika virus (ZIKV) has recently emerged as a critical mosquito-borne pathogen, especially after outbreaks in the Eastern Pacific region.1,2 Concurrent infections with Dengue (DENV) and Chikungunya (CHIKV) virus have posed a significant burden for health care services, and new clinical challenges have been associated with these infections, in particular Guillain-Barré Syndrome, meningoencephalitis, autoimmune manifestations and, more recently, congenital abnormalities.3–10 Since the first isolation of ZIKV in the Americas on March 26, 2015, an estimated 1.3 million individuals have been infected in northeastern Brazil, with an estimated attack rate of 5.5 cases per 1000.11,12
ZIKV infection typically causes a self-limiting, Dengue-like, illness characterized by cutaneous rash, low-grade fever, conjunctivitis, and arthralgia.6 Many reports on ZIKV in the literature describe clinical manifestations based on empirical evidence, since diagnosis is dependent either on virus detection using reverse transcriptase-polymerase chain reaction (PCR) or on plaque reduction neutralization testing, both of which are typically unavailable in most clinical laboratories in affected areas.1 Even in investigations conducted during outbreaks, the majority of patients only tested positive in the first three days of illness, with decreasing sensitivity of PCR observed after this initial period.13
There is a paucity of clinical studies evaluating acute ZIKV infection in the literature and to date no case-series have extensively reported an in depth analysis of the clinical and laboratory abnormalities seen in patients with ZIKV as well as co-infections with DENV or CHKV. A comprehensive understanding of the clinical and laboratory parameters specific to each of these diseases is urgently needed.
As of March 2015, the municipality of Salvador, located in the state of Bahia-Brazil, has witnessed an increasing number of adult patients with Dengue-like symptoms seeking medical assistance at emergency departments throughout the city.12 The present study aimed to describe the clinical manifestations and laboratory findings of patients with confirmed acute ZIKV infection seen at a tertiary care facility in Salvador from 2015 through 2017.
MethodsThe present cross-sectional study was conducted at the emergency department of a 210-bed tertiary care facility of a private hospital at Salvador, Bahia, Brazil from May 27, 2015 through August 31, 2017. All patients aged 18 years or older who sought emergency services and presented symptoms compatible with an infectious clinical condition [systemic, respiratory, gastrointestinal (GI), urinary (UTI) or skin/soft tissue (SSTI)] were triaged by a nurse and then examined by an on-duty physician. Patients were considered eligible if they presented with at least one of the following acute (< 7 days) symptoms: fever (reported or measured), myalgia, arthralgia, rash or headache.
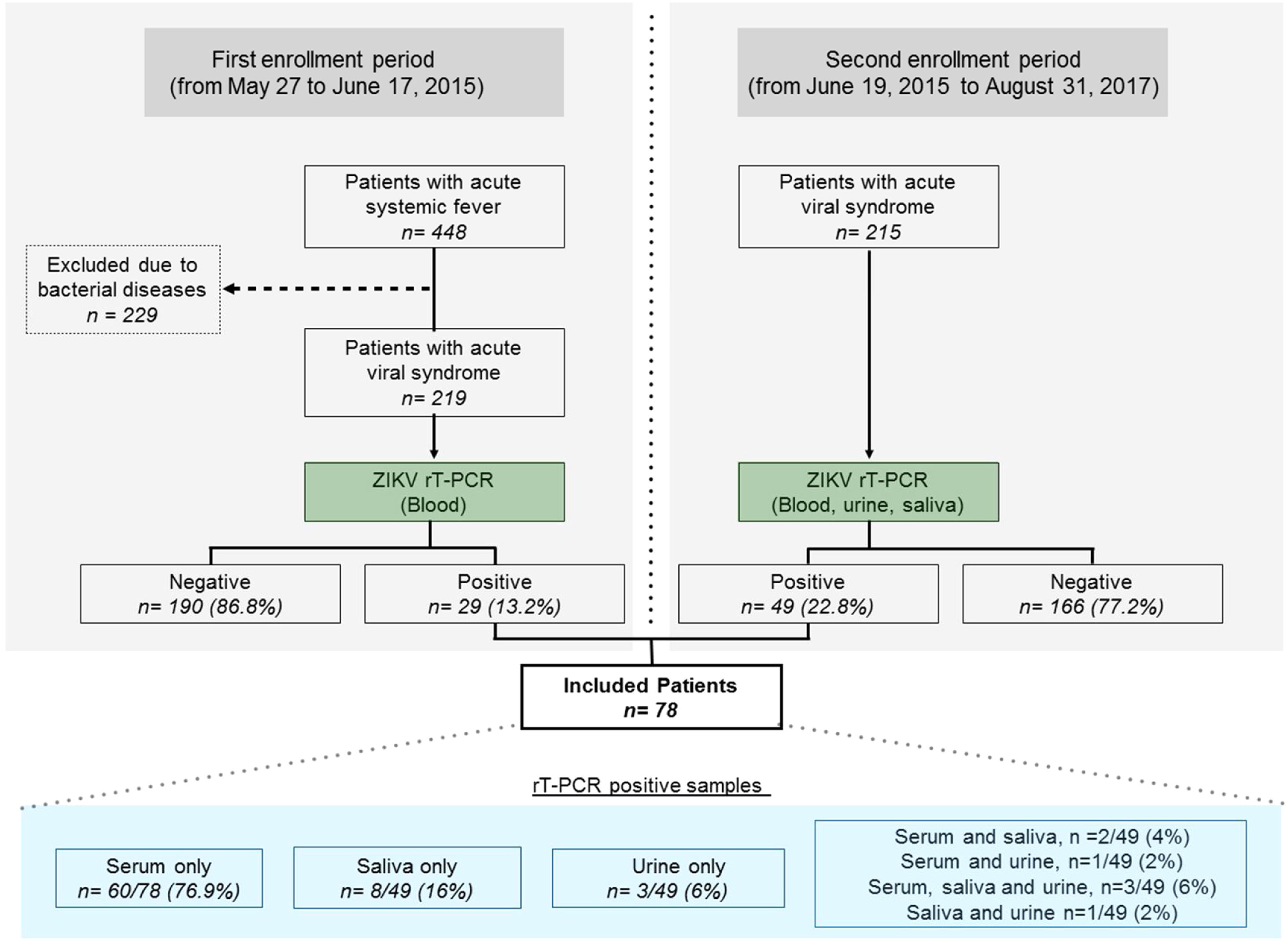
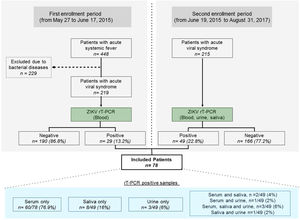
During the initial study period, from May 27 to June 17, 2015, all patients screened for infectious conditions were seen and oriented by a physician specialized in infectious diseases, and blood samples were collected to perform ZIKV diagnosis (Fig. 1). From June 19, 2015 until the end of the study, in addition to blood, urine and saliva samples were also collected. All samples were submitted to viral RNA extraction (Qiagen, USA) and amplified by reverse transcription polymerase chain reaction (PCR) using primers for CHIKV and ZIKV according to previously described protocols.13–15 Dengue fever was diagnosed using a rapid test to demonstrate the presence of IgM and IgG antibodies and/or Dengue NS1 antigen (ECO Diagnostica, Brasil). Patients were selected if a serum, urine or saliva sample tested positive for the presence of ZIKV by molecular diagnosis. All molecular tests were done at the Virology Laboratory, Federal University of Bahia. Any individuals diagnosed with bacterial disease or infectious diseases other than ZIKV were excluded. Blood, urinary, and radiological exams were ordered at the discretion of the attending physician for diagnostic purposes.
The following demographic and clinical variables were evaluated: sex, age, location of residence, history of recent travel to a country with confirmed ZIKV circulation, time (in days) of onset of initial symptoms, rash, myalgia, arthralgia, headache, arthritis (including the involvement of specific joints), conjunctivitis, lymphadenopathy, nausea, diarrhea, dizziness, muscle weakness, axillary temperature, heart and respiratory rate, blood pressure, in addition to any other symptoms and/or abnormal signs. The laboratory parameters investigated were: complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine phosphokinase (CK). Lymphopenia was defined as the total lymphocyte count under 1000 per mm3 and hemoconcentration as a hematocrit level above 47%.
The present study was conducted in accordance with the Good Clinical Practice guidelines and received approval from the Institutional Review Board of Oswaldo Cruz Foundation (protocol numbers 1.159.814 and 1.593.256/CAAE 55882016.6.0000.0040). Written informed consent was obtained from all patients who agreed to participate.
ResultsDuring the first epidemic period from May 27 to June 17, 2015, a total of 448 patients were triaged. An acute systemic viral illness was diagnosed in 219 (48.9%) out of 448 patients (Fig. 1), with 29 (13.2%) testing positive for the presence of ZIKV on molecular testing. Subsequently, in the second period of the study, 49 out of 215 eligible patients (22.8%) with systemic febrile illness tested positive for the presence of ZIKV. The final sample comprised 78/434 (18.0%) ZIKV-infected individuals screened throughout the entire study period. Fifty-two patients were female (66.7%), with a mean age of 38 years. Using molecular diagnostics, 66 patients tested positive for the presence of ZIKV in serum, with two patients also presenting virus in saliva and one in urine, whereas three patients were positive in both saliva and urine. In addition, eight patients without viral detection in serum presented positivity in saliva only, while three were positive in urine only, and one in both saliva and urine (Fig. 2). Altogether, 12/49 (24.5%) of the patients tested positive in saliva or urine (or both) and had no detectable levels of ZIKV in serum.
Four patients (5.1%) were co-infected with CHIKV at the time of the diagnosis of ZIKV: three tested positive for CHIKV in serum, whereas the other was positive only in saliva. Dengue rapid testing indicated that out of 67 ZIKV-infected patients tested, one patient (1.5%) presented DEN-specific IgM and 13 patients (19.4%) had IgG antibodies in serum. Just one patient (1.5%) tested positive for the presence of the Dengue NS1 antigen.
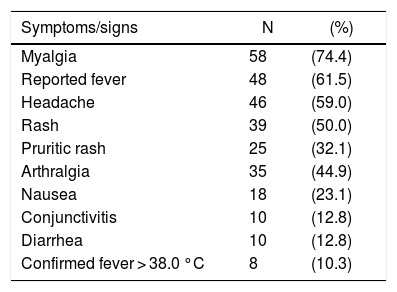
The median time between the onset of symptoms and sample collection was three days (range 1–10 days). The frequency of clinical signs and symptoms are shown in Table 1. Most patients complained of myalgia (74.4%), fever (61.5%), headache (59.0%), rash (51.3%) and arthralgia (44.9%), consistent with the classical presentation of ZIKV. Some patients also reported other rather unusual symptoms, such as nausea (23.1%), vomiting (12.8%), diarrhea (12.8%), dizziness (6.4%) and sore throat (6.4%).
Distribution of reported and observed signs and symptoms of acute Zika infection among 78 patients seen in an emergency room setting.
| Symptoms/signs | N | (%) |
|---|---|---|
| Myalgia | 58 | (74.4) |
| Reported fever | 48 | (61.5) |
| Headache | 46 | (59.0) |
| Rash | 39 | (50.0) |
| Pruritic rash | 25 | (32.1) |
| Arthralgia | 35 | (44.9) |
| Nausea | 18 | (23.1) |
| Conjunctivitis | 10 | (12.8) |
| Diarrhea | 10 | (12.8) |
| Confirmed fever > 38.0 °C | 8 | (10.3) |
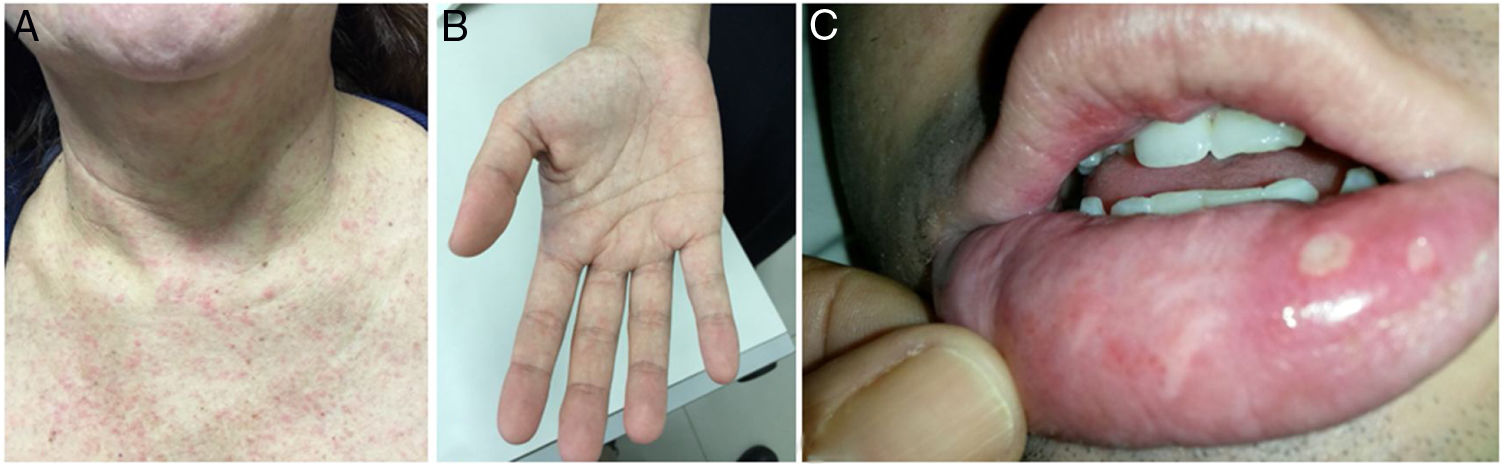
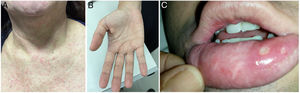
At the time of admission in the emergency room, 39 patients (50.0%) presented with a cutaneous rash (Fig. 2A) and some with a palmar rash (Fig. 2B). Other less frequent clinical findings were conjunctivitis (10/78, 12.8%), cervical lymphadenopathy (9/78, 11.5%), body temperature >38 °C (8/78, 10.3%) and tachycardia, which was found in 8/78 patients (10.3%). It is worth noting that five patients had ankle edema, and four had edema and pain in their wrists and hands. Two of these patients were also found to be co-infected with CHIKV. Two patients presented painful bilateral inguinal lymphadenopathy. Oral ulcers were observed in one patient co-infected with CHIKV (Fig. 2C). The classical presentation of acute viral infection with fever, myalgia, arthralgia and rash was observed in only 13 patients (16.7%), while 19 patients (24.4%) presented with fever, myalgia and rash, but without arthralgia. The patient who tested positive for NS1 for DENV had a 4-day history of myalgia, rash, and headache, but was not tested for DENV by PCR.
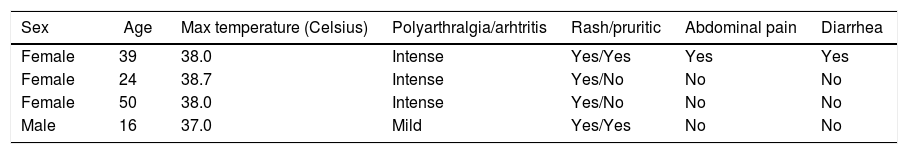
Signs and symptoms in coinfected patients with Zika and Chikungunya are shown in Table 2. Articular involvement predominated in all patients with intense polyarthritis in three.
Distribution of signs and symptoms in co-infected patients with acute Zika and Chikungunya.
| Sex | Age | Max temperature (Celsius) | Polyarthralgia/arhtritis | Rash/pruritic | Abdominal pain | Diarrhea |
|---|---|---|---|---|---|---|
| Female | 39 | 38.0 | Intense | Yes/Yes | Yes | Yes |
| Female | 24 | 38.7 | Intense | Yes/No | No | No |
| Female | 50 | 38.0 | Intense | Yes/No | No | No |
| Male | 16 | 37.0 | Mild | Yes/Yes | No | No |
Laboratory findings of the included patients are presented in Table 3. A CBC was ordered for 76 patients. Four patients with ZIKV infection (5.3%) presented with hemoconcentration. Total leukocyte counts ranged from 1340 to 21,250 cells per mm3 with a median of 5505 cells per mm3. Ten patients (13.2%) had a total leukocyte count above 10,000 cells per mm3, with two patients presenting leukocytosis with over 20,000 leukocytes per mm3. The first, a 24 yr-old woman, reported fever and chills one day before admission, with no gastrointestinal, respiratory, urinary, or cutaneous symptoms. Her total leukocyte count was 20,210 cells per mm3 with 1% atypical lymphocytes and 2% band forms. The second patient, a 23 yr-old pregnant woman at five weeks of gestation at the time of diagnosis, complained of a prior pruritic rash one day earlier, which lasted 24 h. She was taking corticosteroids at the time of her emergency room visit. Her total leukocyte count was 21,250 cells per mm3, with 1% band forms and 11% atypical lymphocytes. She gave birth to a normal baby with no neurological abnormalities. Both patients presented negative blood cultures and both totally recovered a few days later. All patients were discharged without complications.
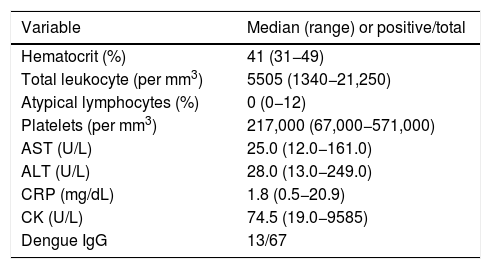
Laboratory markers among 78 patients with acute Zika infection seen in an emergency room setting.
| Variable | Median (range) or positive/total |
|---|---|
| Hematocrit (%) | 41 (31−49) |
| Total leukocyte (per mm3) | 5505 (1340−21,250) |
| Atypical lymphocytes (%) | 0 (0−12) |
| Platelets (per mm3) | 217,000 (67,000−571,000) |
| AST (U/L) | 25.0 (12.0−161.0) |
| ALT (U/L) | 28.0 (13.0−249.0) |
| CRP (mg/dL) | 1.8 (0.5−20.9) |
| CK (U/L) | 74.5 (19.0−9585) |
| Dengue IgG | 13/67 |
AST (aspartate aminotransferase), ALT (alanine aminotransferase), CRP (C-reactive protein). CK (creatine phosphokinase).
Lymphopenia (less than 1000 per mm3) was observed in 26 patients (34.2%) and atypical lymphocytes (ranging from 1% to 12%) were found in 23 patients (30.3%). Twelve patients (15.8%) had a total monocyte count above 900 cells/mm3, and four (5.3%) below 200 cells/mm3. Three patients (3.9%) had band forms greater than 2%. Platelet count below 100,000 cells/mm3 was seen in one patient (1.3%).
Results of C-reactive protein (CRP) were obtained for 71 patients, median 1.8 mg/dL (ranging from 0.5 to 20.9 mg/dL). In eight patients (11.3%), CRP levels were >5.0 mg/dL, and in the four >10.0 mg/dL. One of the latter patients was a 61 yr-old man with type II diabetes who presented with fever lasting four days, as well as chills and diarrhea. His CRP level was 20.9 mg/dL and total leukocyte count was 2800 cells/mm3 (0% band forms and 0% atypical cells.) A transient elevation of liver enzymes was observed, evidenced by AST of 161 U/L and ALT of 249 U/L. The patient had no organ dysfunction. The second patient was a 21 yr-old man with four-day asthenia, arthralgia, fever, headache, and myalgia. His CRP level was 15.6 mg/dL, total leukocyte count was 6600 cells/mm3, with 0% band forms and normal liver enzymes. Another patient was a 24 yr-old woman with 1-day history of fever and chills, with no other complaints. Her CRP value was 13.8 mg/dL and total leukocyte count was 20,210 with 2% band forms. She had normal liver enzyme levels (AST of 18 U/L and ALT of 23 U/L). Finally, the fourth patient was a 22 yr-old woman with a four-day history of myalgia and fever, who, after two days, began experiencing a non-productive cough. Her CRP level was 13.4 mg/dL, and total leukocyte count was 6940 cells/mm3, with 0% band forms. All four patients recovered in a few days with an uneventful course. For all patients, blood cultures showed negative results.
AST and ALT liver enzyme levels, tested in 63 patients, showed a median value of 25 U/L (ranging from 12 to 161 U/L) and 28 U/L (ranging from 913 to 249 U/L), respectively. Only two patients (3.2%) had elevated AST levels above 100 U/L. CK levels were ordered for 24 patients. Four patients (16.7%) had values > 175 U/L and one patient had a CK of 9585 U/L. This patient was a 41 yr-old man with a three-day history of intense pain in back, thighs, and calves. The presence of ZIKA was positive in saliva only.
DiscussionThe results of the present study showed that during the recent ZIKV outbreak in the city of Salvador, Brazil, 18% of patients with acute viral syndrome were confirmed with ZIKV infection by molecular testing. The most frequently reported clinical symptoms, myalgia, fever, and low-grade fever, were mild and non-specific. Laboratory markers also did not show significant alterations. In addition, co-infection with CHIKV was present in 5% of the ZIKV-infected patients.
Our results corroborate those reported by researchers on Yap Island, who estimated that just one in six patients with ZIKV-confirmed infection presented supposedly classical symptoms of Zika fever, i.e. a combination of a rash, fever and conjunctivitis.1 In addition, these symptoms were observed in just 26.2% of ZIKV-positive blood donors between 3 to 10 days after blood donation during the French Polynesia epidemic.16 Our results are similar to findings reported in patients diagnosed with acute ZIKV infection in 2016 from Araraquara and Ribeirão Preto (São Paulo), as well as Rio de Janeiro, Brazil.17–19 A similar distribution of sex, age, and clinical findings, such as myalgia, headache, and conjunctivitis, was found between these three studies and our results.
In contrast to the signs and symptoms of ZIKV infection described herein, DENV-infected patients more often present classical symptoms, which may aid in performing differential diagnosis in clinical settings. Indeed, during outbreaks of Dengue fever in Kenya and in Ecuador, almost all patients had fever, headache, arthralgia and myalgia. In each of these settings, 67% and 20% of patients required hospitalization, respectively, which is a very distinct outcome not seen in our patients.18,20
The moderate rate (18%) of confirmed ZIKV diagnosis in the patients considered herein is 10-fold higher than that reported by Silva et al., who confirmed ZIKV infection in 1.4% of febrile patients seen at a public health unit in Salvador between 2014 and 2016.21 This discrepancy may be explained by the fact that these authors conducted surveillance for arboviral diseases for a longer period than our study, prior to the outbreak in Bahia in 2015.
Regarding laboratory findings, the majority of our patients presented normal CBC, while hemoconcentration was observed in 5.3% and only one patient presented thrombocytopenia. This contrasts to patients with Dengue fever, who more frequently experience hemoconcentration and thrombocytopenia. The ZIKV studied patients did not present non-specific elevated levels of liver enzymes, and less than 5% had AST levels higher than 100 U/L. In comparison to Dengue fever, these findings may help differentiate ZIKV-infected patients from those with DENV in an acute setting, and can provide indications of a clinical diagnosis in the absence of hemoconcentration and thrombocytopenia. A study conducted in midwestern Brazil observed differences in laboratory parameters among patients with Dengue and Zika infection. Azeredo et al. demonstrated that patients infected with DENV alone presented higher ALT levels compared to patients with acute ZIKV infection.22 In South Australia, thrombocytopenia was seen in 49.0% of patients with Dengue fever compared to just 1.3% of the patients in the present study.23
Yan et al. presented simple clinical and laboratory assessments for distinguishing between Zika infection and Dengue fever.24 Non-purulent conjunctivitis and normal monocyte and platelet counts were the main parameters used to establish a diagnosis of ZIKV.
With respect to coinfection, 5.1% of the ZIKV-infected patients also presented evidence of CHIKV infection. However, no differences in clinical or laboratory markers were seen in these coinfected patients. Pruritic rash which occurred in half of the coinfected patients could help in adding clinical suspicion of ZIKV infection in patients presenting with fever and polyarthralgia. Herein, 19.4% the studied patients presented serological evidence of past DENV infection, and just one patient had a positive Dengue NS1 test result. Of note, a false-positive DENV NS1 antigen test observed in the context of acute ZIKV infection might help to explain this result, as proposed by Gyurecha et al.25
Our study proposes that health professionals consider the following clinical manifestations when attempting to establish a diagnosis of acute ZIKV infection: non-specific acute symptoms, e.g. myalgia, arthralgia, headache; variable physical signs that may include a pruritic rash and fever; and lab results ranging from normal levels of hematocrit and platelets to mild liver enzyme levels. Although the intent of the present study was not to design a protocol for the differential diagnosis of ZIKV, the authors feel that a future investigation should take into account the above-mentioned variables.
With respect to ZIKV laboratory diagnostics, PCR positivity was mainly observed in blood samples (85% of cases), while saliva (29%) and urine (8%) positivity was lower than that reported in other studies.26,27 It is important to consider that ZIKV RNA may remain present in urine samples for more than three weeks after the onset of symptoms,28 which may help explain the discrepancy in urine analysis. Accordingly, it could be advantageous to simultaneously test multiple tissue types in order to more accurately reveal the true rate of virus detection.
The present study suffers from several limitations. Patients were screened at a hospital emergency service and some may have been discharged prior to recruitment, especially in the case of mild symptoms or fever. It was also conducted in a private hospital where the selected sample does not come from the general population with a bias towards a better-nourished population. However, since this study was conducted during the epidemic peak of ZIKV in northeastern Brazil, it was possible to include a significant number of patients with acute ZIKV infection. It is also important to note that during the epidemic peak period, PCR analysis for ZIKV was primarily conducted in blood samples, which may have negatively influenced the accurate identification of ZIKV-positive patients, especially in the early enrollment period. Although there were no control patients included herein, we are confident that no patients were misclassified, as this cross-sectional study aimed to describe clinical and laboratory characteristics exclusively in PCR-positive patients.
In conclusion, the diagnosis of ZIKV infection will remain a difficult task for clinicians in acute settings where molecular testing is not available, thus necessitating the reliance on clinical examination and laboratory tests. In addition to increasing access to rapid ZIKV and DENV diagnostic tests, more studies are also needed to provide clinicians with better algorithms based on symptoms and biomarkers to facilitate differential diagnoses with respect to other arboviral infections.
FundingThis work was supported by the European Union’s Horizon 2020 Research and Innovation Programme under ZIKAlliance Grant Agreement no. 734548.
The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Conflicts of interestThe authors declare no conflicts of interest.
Grassi, M.F. is currently receiving a scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Nacional de Desenvolvimento do Ensino Superior Particular (FUNADESP). The authors are grateful to Andris K. Walter for English language revision and copyediting services.