Nosocomial invasive infections due to Acinetobacter species are gradually increasing in the health care settings especially in intensive care units (ICUs).1
This retrospective study is designed to investigate risk factors associated with Acinetobacter bacteremia among children hospitalized in Dr. Behçet Uz Children's Hospital, during 2005–2011. Nosocomial bacteremia was defined as isolation of Acinetobacter spp. obtained from blood 72h after admission to the hospital in the presence of infection. The control group was randomly recruited from the age-matched patients who had no fever or clinical deterioration after the hospitalization more than 72h.
Identification of Acinetobacter spp. in blood samples was performed using a Bact-Alert (bioMérieux, France) automated system. Susceptibility of the isolates by determination of the minimum inhibitory concentrations was performed by VITEK2 (bioMérieux, France) compact system.
The χ2 and Fisher's exact test were used to evaluate the association between categorical quantitative variables and Student's t-test or Mann–Whitney-U test for continuous variables.
A total of 35 patients and 70 control patients without bacteremia were included in this retrospective case–control study. The patients had the following diagnosis: primary bacteremia 9 (25.7%), pneumonia 9 (25.7%), central-catheter related infection 7 (20%), peritonitis, urinary tract infection, cerebrospinal fluid infection, and post-burn wound infection.
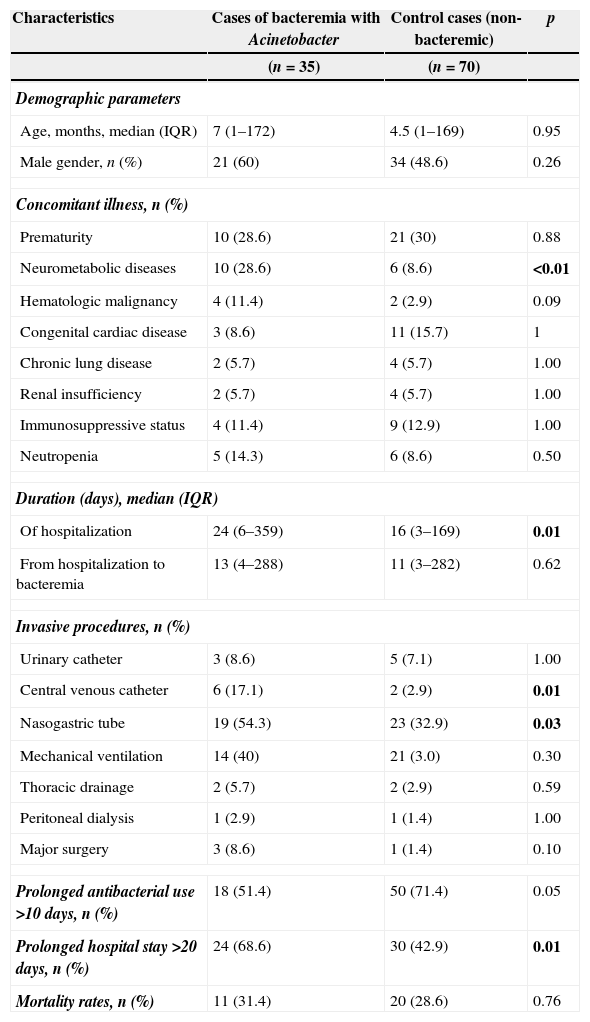
There was no significant difference in terms of gender, age, prior history of hospitalization or concomitant illness except underlying neurometabolic disease (p>0.05). Length of hospital stay was statistically higher in patients with bacteremia than in control patients [24 days (6–359) for patients, 16 days (3–169) for controls (p=0.01)]. Further analysis indicated that presence of central venous catheter, nasogastric tube, prolonged use of antibiotics, hospitalization for >10 days, and concomitant neurometabolic disease (p<0.05) were significantly more frequent among patients compared to the control group (p<0.05) (Table 1).
Cases of bacteremia×control cases (non-bacteremia).
| Characteristics | Cases of bacteremia with Acinetobacter | Control cases (non-bacteremic) | p |
|---|---|---|---|
| (n=35) | (n=70) | ||
| Demographic parameters | |||
| Age, months, median (IQR) | 7 (1–172) | 4.5 (1–169) | 0.95 |
| Male gender, n (%) | 21 (60) | 34 (48.6) | 0.26 |
| Concomitant illness, n (%) | |||
| Prematurity | 10 (28.6) | 21 (30) | 0.88 |
| Neurometabolic diseases | 10 (28.6) | 6 (8.6) | <0.01 |
| Hematologic malignancy | 4 (11.4) | 2 (2.9) | 0.09 |
| Congenital cardiac disease | 3 (8.6) | 11 (15.7) | 1 |
| Chronic lung disease | 2 (5.7) | 4 (5.7) | 1.00 |
| Renal insufficiency | 2 (5.7) | 4 (5.7) | 1.00 |
| Immunosuppressive status | 4 (11.4) | 9 (12.9) | 1.00 |
| Neutropenia | 5 (14.3) | 6 (8.6) | 0.50 |
| Duration (days), median (IQR) | |||
| Of hospitalization | 24 (6–359) | 16 (3–169) | 0.01 |
| From hospitalization to bacteremia | 13 (4–288) | 11 (3–282) | 0.62 |
| Invasive procedures, n (%) | |||
| Urinary catheter | 3 (8.6) | 5 (7.1) | 1.00 |
| Central venous catheter | 6 (17.1) | 2 (2.9) | 0.01 |
| Nasogastric tube | 19 (54.3) | 23 (32.9) | 0.03 |
| Mechanical ventilation | 14 (40) | 21 (3.0) | 0.30 |
| Thoracic drainage | 2 (5.7) | 2 (2.9) | 0.59 |
| Peritoneal dialysis | 1 (2.9) | 1 (1.4) | 1.00 |
| Major surgery | 3 (8.6) | 1 (1.4) | 0.10 |
| Prolonged antibacterial use >10 days, n (%) | 18 (51.4) | 50 (71.4) | 0.05 |
| Prolonged hospital stay >20 days, n (%) | 24 (68.6) | 30 (42.9) | 0.01 |
| Mortality rates, n (%) | 11 (31.4) | 20 (28.6) | 0.76 |
Risk factors of mortality in Acinetobacter bacteremia are male gender, prior use of carbapenems and glycopeptides antibiotics, mechanical ventilation and gentamicin resistance of the isolate.
Several studies reported the risk factors for Acinetobacter bacteremia especially in ICUs. Among these studies, length of stay in ICU, previous use of antimicrobials, mechanical ventilation, male gender, neurologic impairment, prior colonization, and presence of excess intravascular devices were found to be independent risk factors for bacteremia.2–5 In our study, the ratio of children having central venous catheter, nasogastric tube and concomitant neurometabolic disease were found to be higher compared to control group. Plus the Acinetobacter group was found to have longer length of hospitalization and longer antibiotic usage, compared to control group (p>0.05).
Central venous catheter and nasogastric tube insertions were found to be risk factors for bacteremia. Central venous catheterization was inserted in 17.1% of the patients with Acinetobacter bacteremia, and in only 2.9% of the control group (p=0.01).
In conclusion central venous catheter insertion, long length of stay, prolonged use of antibiotics, and concomitant neurometabolic disease were risk factors for the presence of bacteremia. Male gender, prior use of carbapenems and glycopeptides antibiotics, use of mechanical ventilation and gentamicin resistance of the isolates were also found to be risk factors of mortality in Acinetobacter bacteremia.
Conflict of interestThe authors declare no conflict of interest.