Acinetobacter baumannii is a major nosocomial pathogen and carbapenems are the mainstream agents against these organisms.1 However, carbapenem-resistant A. baumannii (CRAB) isolates have become widely disseminated owing to the emergence of Class D carbapenemases, mainly OXA-23, which are highly prevalent in Brazil.1
Polymyxins B and colistin are often the last therapeutic options against CRAB isolates.2 The combination of polymyxins with another antimicrobial has been proposed to overcome some shortcomings of polymyxins in monotherapy, including the potential for the emergence of hetero-resistant subpopulations and a possible limited bactericidal activity in vivo, considering recent pharmacokinetics studies.2
In vitro studies may provide some support defining that candidate antimicrobials would more likely present a beneficial effect when combined with polymyxins. We evaluated the combination of non-bactericidal concentrations of polymyxin B, which are also more likely to be clinically achieved,2 with other antimicrobials in concentrations usually reached with common dosage regimes against OXA-23-producing A. baumannii isolates.
Two distinct CRAB strains positive for blaOXA-51 and blaOXA-23 genes, as characterized elsewhere,1 recovered from patients admitted at Porto Alegre hospitals between 2007 and 2008 were selected. MICs of antimicrobials were determined by broth microdilution method according to the Clinical and Laboratory Standards Institute.3
Time-kill studies (TKS) with polymyxin B at 1× MIC for strain 01, and 1/4, 1/2 and 1× MIC for strain 02 were performed in combination with fixed concentrations of imipenem (4mg/L), meropenem (4mg/L), rifampicin (1mg/L), tigecycline (1mg/L), amikacin (16mg/L) and ceftazidime (mg/L). All experiments were performed in duplicate.
Tubes with freshly cation-adjusted Muller-Hinton broth (CAMHB) with no antibiotic (growth control) and with the antibiotic concentrations described above, alone or in combination, were prepared. Colonies were suspended in 5mL of CAMHB and were allowed to grow to a log phase. The final inoculum of approximately 5×105 colony-forming units (CFU)/mL containing the drugs was incubated in a shaking water bath at 35°C in ambient air. Samples were removed from each bottle at 0, 30min, 1, 4, 12 and 24h. Serial 10-fold dilutions (10−1 to 10−8) were performed in 0.9% sterile sodium chloride solution. Aliquots of 10μL were plated on agar plate with 5% sheep blood. Plates were incubated for 24h at 35°C.4 Colony counts were averaged between the duplicate plates for each experiment. A growth control was included with each isolated tested. Quality control was performed using Pseudomonas aeruginosa ATCC 27853 and Enterococcus faecalis ATCC 29212.
The MICs of the strains 01 and 02, respectively, for the following drugs were 0.25 and 2mg/L for polymyxin B, 32 and 16mg/L for imipenem and >512 and 16mg/L for amikacin. Both strains had MICs of 32 for meropenem and ceftazidime, ≤0.0625 for tigecycline and ≥32mg/L for rifampicin.
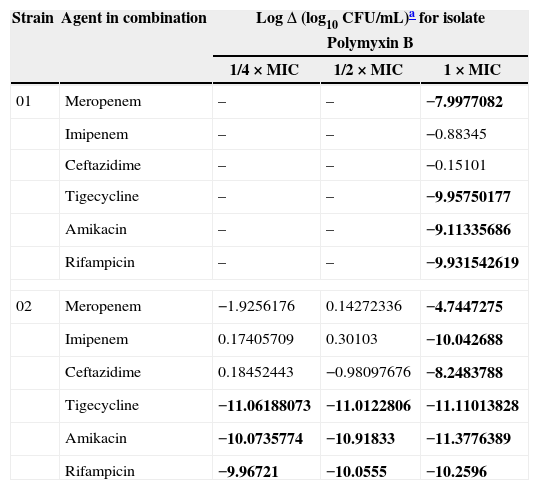
In TKS, no antimicrobial alone showed bactericidal activity against any of the CRAB strains. The 24-h changes in bacterial count after exposure to polymyxin B combined with other antimicrobials of the two CRAB strains are displayed in Table 1.
The 24h change in bacterial count after exposure to polymyxin B combined with other antimicrobials against the two strains of carbapenem-resistant Acinetobacter baumannii.
| Strain | Agent in combination | LogΔ (log10CFU/mL)a for isolate | ||
|---|---|---|---|---|
| Polymyxin B | ||||
| 1/4×MIC | 1/2×MIC | 1×MIC | ||
| 01 | Meropenem | – | – | −7.9977082 |
| Imipenem | – | – | −0.88345 | |
| Ceftazidime | – | – | −0.15101 | |
| Tigecycline | – | – | −9.95750177 | |
| Amikacin | – | – | −9.11335686 | |
| Rifampicin | – | – | −9.931542619 | |
| 02 | Meropenem | −1.9256176 | 0.14272336 | −4.7447275 |
| Imipenem | 0.17405709 | 0.30103 | −10.042688 | |
| Ceftazidime | 0.18452443 | −0.98097676 | −8.2483788 | |
| Tigecycline | −11.06188073 | −11.0122806 | −11.11013828 | |
| Amikacin | −10.0735774 | −10.91833 | −11.3776389 | |
| Rifampicin | −9.96721 | −10.0555 | −10.2596 | |
–, not performed.
LogΔ (final inoculum of the combined drugs−final inoculum of the most active drug in combination [log10CFU/mL]). Synergy highlighted in bold was defined as a ≥2log10 decrease in colony count after 24h by the combination compared with the most active single agent. Indifference was defined as a <2log10 increase or decrease in colony count at 24h by the combination compared with that by the most active drug alone. Antagonism was defined as a ≥2log10 increase in colony count after 24h by the combination compared with the most active drug alone. Bactericidal activity was defined as a ≥3log10 decrease in colony count after 24h.4
Our study showed that either of tigecycline, amikacin or rifampicin could make the most active agent in combination with polymyxin B, showing bactericidal effects against both strains, even at subinhibitory concentrations of polymyxin B against one strain. Notably, both isolates were highly resistant to amikacin and rifampicin, but when these drugs at concentrations lower than their MICs were combined with polymyxin B, a strong bactericidal activity was detected. Synergism between rifampicin and polymyxin B has been previously demonstrated by TKS,4 but no study had assessed the combination of amikacin with polymyxin B against A. baumannii.
The combination of tigecycline with polymyxin B was highly bactericidal against both isolates, even at subinhibitory concentration of the latter drug for one isolate. Actually, tigecycline MICs of our CRAB isolates were very low, but tigecycline presents only bacteriostatic activity in vitro. The combination of this drug with polymyxins has been previously assessed against Enterobacteriaceae and only in one experimental study with A. baumannii, with synergistic effects demonstrated.5
Beta-lactams agents were less effective in our study in combination with polymyxin B, with no drug showing synergistic effect when combined with subinhibitory concentration of polymyxin B. Imipenem and ceftazidime were only synergistic for strain 2.
Although our study was limited by the evaluation of only two strains, it assessed the combination of polymyxin B with several classes of drugs, only at non-bactericidal concentrations of each drug alone. It also provided data about OXA-23-producing isolates, which is the most common mechanism determining carbapenem resistance in A. baumannii worldwide.1
In summary, tigecycline, amikacin and rifampicin were the most active agents in combination with polymyxin B, as demonstrated by a strong bactericidal effect even at subinhibitory concentration of the latter. These agents may show a beneficial effect when combined with polymyxin B in the treatment of OXA-23-producing CRAB and it warrants confirmation in further experimental and clinical studies.
Conflict of interestA. P. Z. is a research fellow from CNPq (305263/2011-0) and has received consultancy fees from Pfizer, Eurofarma and Forest Laboratories. All other authors: none to declare.
We are very grateful to Dr. Andreza F. Martins for kindly providing the isolates for this study. This study was supported by Fundo de Incentivo a Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (11-0252) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (11/0898-3).