To determine the prevalence of HPV and the risky sexual behaviors associated to it in a sample of male college students, taking into account genotype and viral load.
MethodsFrom 2002 to 2003, male students from the Autonomous University of Morelos State completed a questionnaire and provided self-collected genital samples to detect and quantify HPV. We performed a bivariate and a multivariate logistic regression analysis to identify correlates associated with the infection and to assess the viral load as a function of the viral infecting type. The fragments of β-globin gene and L1 of HPV, were amplified, purified and cloned, to evaluate viral load.
ResultsAmong 253 subjects, HPV prevalence was 19.4%, and HPV16 was the most common subtype. History of STIs (OR=4.8; 95% CI 1.2–18.9), contraceptive pill use by female partner (OR=2.6; 95% CI 1.1–6.3) and exchanging sex for money (OR=4.9; 95% CI 1.2–20) were associated to the HPV infection. HPV16 viral load was 7.8 copies (HPV/beta-globin) compared to 0.9 copies for other HPV types.
DiscussionHPV16 displayed the highest viral load, and it was the most prevalent. It was found that using contraceptive pills by female partners was associated with HPV infection.
HPV is the most common sexually transmitted infection (STI) around the world and the majority of the infections have an asymptomatic course; however, HPV chronic infection may cause cervical, penis or anal cancer.1 It has been proven that men can transmit the infection to their sexual partners; one study in women within three months of sexual debut and with only one sexual partner showed 28.5% of HPV infection after one year of follow up.2 The number of male sexual partners was a major risk for HPV infection.
HPV prevalence among male college students from different countries ranged from 1.3% in Japan,3 8.5% in Mexico,4 10.6% in South Korea,5 25.8% and 33% in Washington State,6,7 42.8% in Hawaii,8 to 84% in Chile.9 Age at sexual debut, number of sexual partners and relationship with sexual workers have been associated with HPV infection among males.10–13 Circumcision and condom use have been reported as protective factors but with inconsistent results10,11,13–16; this could be explained by the different anatomical sampling sites, the HPV detection method and the different population studied.17 Nevertheless, HPV risk factors have been insufficiently studied in young males. Korean male students with sexual debut at 18–19 years old had 4.4 times greater risk of HPV infection than students ≥20 years old at sexual debut.5 Students from the USA with past smoking displayed 1.6 times greater risk of HPV infection than students that had never smoked, and students with ≥ one sexual partner during the last four months had 2 times greater risk of HPV infection than students without sexual partners,6 and African young men with ≥ two female sexual partners during last year had 1.6 times greater risk of HPV infection.16
These studies have evaluated demographic and sexual behavior characteristics; however, it is also necessary to study viral factors, i.e. the viral load, because it points to efficient viral transmission, as has been the case in relation to HIV and Herpes Simplex Virus (HSV).18 In fact, HPV viral load has been associated with high-grade cervical lesions.19–21 Nevertheless, the relation between HPV infection and viral load in men is unknown. The present study evaluated the prevalence, risk factors for HPV infection, and viral load in Mexican male college students.
MethodsStudy designThe first and second year male students of Medicine, Psychology and Pharmacy of the Autonomous University of Morelos State, at central Mexico, were invited to participate in August 2002, and male students of first year in August 2003. The project was approved by the bioethics committee from Instituto Nacional de Salud Publica, Mexico. The research team visited each classroom, explained the purpose of the study and invited all male students to participate. It was not the aim to obtain a representative sample of the student population. Afterwards, they signed a consent form, answered a self-applied questionnaire (demographic and sexual behaviors sections) and provided a self-collected genital sample obtained from their penis, glans and sulcus.
Each participant received verbal instructions from trained personnel, so that the sample was directly taken through vigorous rubbing with a sterile swab; if the subject was not circumcised, he had to proceed after retraction of the foreskin. The swab material collected was deposited in a tube with maintenance medium (Digene Specimen Collection Kit, USA), that was then transported immediately to the laboratory and frozen at −20°C until processing. DNA was extracted from the genital samples by Qiagen columns (QIAamp DNA Mini Kit). A fragment of the β-globin gene was first amplified by PCR to check the sample quality with GH20 and PC04 primers.22 The detection of HPV DNA by PCR was carried out with primers MY09/MY1122 only for the β-globin-positive samples. After this stage, all HPV-negative samples were further analyzed with GP5/GP6 primers23 to optimize HPV detection.24 HPV-positive samples with either set of primers were typified by means of a linear array HPV genotyping test (Roche, Branchburg, NJ, USA) and classified as single or multiple infections.
Viral load quantificationThe 268-bp fragments of the β-globin gene and the 450-bp fragments of the HPV L1 gene were amplified by PCR (GH20/PC04 and MY09/MY11 primers sets respectively) and purified by QIAquick Spin (QIAGEN, Germany). Subsequently, fragments were cloned separately using the pCR2.1-TOPO system (TOPO TA Cloning, Invitrogen, USA); finally, the plasmid DNA purification was performed by QIAprep Miniprep (QIAGEN, Germany). HPV plasmid fragment was 4381bp with concentration of 260ng/μL, the β-globin plasmid was 4199bp and 210ng/μL of concentration, considering the molecular weight average 650g/bp, the stock concentration was 5.5×1010copies/μL of HPV and 4.6×1010copies/μL of β-globin. Six dilutions of each plasmid were made in triplicate for each run, ranging from 1:1000 to 1:100,000,000 to generate a standard curve of HPV from 55,120,000 to 551.2copies/μL and a β-globin curve from 46,620,000 to 466.2copies/μL. Determination of the viral load was performed by SYBR Green I real-time PCR (FastStart Master SYBR Green Rox, Roche, Germany). The reaction mixture contained 1× master mix, 1μL of DNA and 0.3pmol of primers GH20/PC04 (β-globin) and 0.5pmol GP5/GP6 (HPV) to a final volume of 15μL. Each sample was run in triplicate, if the coefficient of variation (CV) was higher than 5%, the sample was repeated.
Variable definitionsSexually active students were persons with anal or vaginal intercourses. The variables “warts on the penis” and “previous STIs” were self-reported when filling out the questionnaire. In regard to “frequency of condom use”, the “not always” category was built with the following answers: “most of the time”, “half of the time” or “rarely”. In the case of the “always” category, it was confirmed that the participants answered yes to having used a condom at first sexual intercourse, at some other time in their life, and at last sexual intercourse. Age at sexual debut was stratified as ≤15 years old or ≥16 years old, in function to first quartile; the number of sexual partners was stratified as 1–2 sexual partners or ≥3 sexual partners during last year, in function to third quartile, both variables were not normally distributed. Contraceptive pills used by female partners during last sexual relationships were considered according to the response given by the participants.
Statistical analysisThe factors associated to HPV infection were evaluated using a logistic regression model; backward stepwise elimination method was employed in multivariate analysis, the final model being selected when all variables had p<0.10. Viral load and continuous variables are presented as median and interquartile range (IQR) and the Mann–Whitney test was used to compare subgroups. The statistical analyses were performed using SPSS 15.0 (IBM Company), p-values ≤0.05 were considered as statistically significant.
ResultsWe contacted 450 students, 67.5% (304 students) sexually active, out of these 84.2% (256 students) provided genital samples. Three students were excluded because β-globin was not detected; thus, 253 students were included in the analyses. The median age of the participants was 21 years (IQR 20–23); on average, students had their sexual debut at 17 years old (IQR 15–18) with 18 year old (IQR 16–19) sexual partners. The students mentioned three (IQR 2–6.5) lifetime sexual partners and one (IQR 1–2) during last year. Forty percent of the students reported illegal drug consumption, 4.0% reported a history of STIs, 20% mentioned always using a condom, and more than 10% reported sexual relationship with sexual workers. Table 1 shows the demographic and sexual behavior characteristics.
Demographic, clinical and sexual behavior characteristics in Mexican male college students.
| Variable | n | % |
|---|---|---|
| Age | ||
| 18–20 years | 111 | 43.9 |
| ≥21 years | 142 | 56.1 |
| Illegal drug consumption | ||
| Yes | 102 | 40.3 |
| No | 151 | 59.7 |
| Frequency of alcohol consumptiona | ||
| High | 46 | 18.2 |
| Low | 207 | 81.8 |
| Warts on the penis | ||
| Yes | 55 | 21.7 |
| No | 198 | 78.3 |
| Previous STIs | ||
| Yes | 10 | 4.0 |
| No | 243 | 96.0 |
| Age at sexual debut | ||
| ≤15 years | 66 | 26.1 |
| ≥16 years | 187 | 73.9 |
| Time to knowing first sexual partner | ||
| 1 day–1 month | 92 | 36.4 |
| ≥2 months | 161 | 63.6 |
| Sexual partners during the previous year | ||
| ≥3 | 44 | 17.4 |
| 1–2 | 209 | 82.6 |
| Frequency of condom use | ||
| Always | 58 | 22.9 |
| Not always | 195 | 77.1 |
| Contraceptive pill use during last intercourse | ||
| Yes | 27 | 10.7 |
| No | 226 | 89.3 |
| Exchanging sex for money | ||
| Yes | 10 | 4.0 |
| No | 243 | 96.0 |
| Intercourse with sexual workers | ||
| Yes | 28 | 11.1 |
| No | 225 | 88.9 |
| Same sex partners | ||
| Yes | 23 | 9.1 |
| No | 230 | 90.9 |
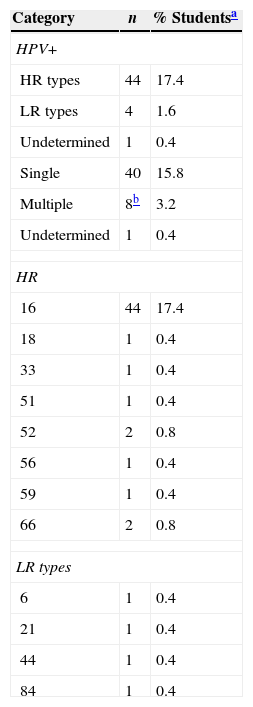
Forty nine samples were detected as HPV-positive (34 with MY primers and 15 with GP primers), yielding an HPV prevalence of 19.4% (95% CI 14.5–24.2). High-risk genotypes prevalence was 17.4% (95% CI 12.7–22.1), low-risk genotypes 1.6%, (95% CI 0.0–3.1) and one sample remained undetermined. Table 2 shows the different HPV genotypes detected, multiple infection was detected in 3.2% of the students (seven students with two types and one student with three types). HPV16 was the most prevalent in the male college students analyzed (17.4%, 95% CI 12.7–22.1).
Type of HPV infection in Mexican male college students.
The β-globin copies/μL among the samples ranged from 35,721 to 10,791,496 and the HPV copies/μL ranged from 14,348 to 81,376,686. The viral load was normalized (HPV copies/μL/β-globin copies/μL), the minimum value was 0.40 and the maximum value 37.6, with a median of 7.1 (IQR 2.1–11.3 HPV/β-globin). Single infection (median=6.6; IQR, 2.6–11.5) in comparison with multiple infection (median=10; IQR, 3.1–11.3) was not different (p=0.894, Mann–Whitney test). However, the HPV16-positive samples had 7.8 HPV/β-globin (IQR, 4.5–11.4) higher than other HPV types which displayed 0.9 HPV/β-globin (IQR 0.5–10.6), (p=0.034, Mann–Whitney test).
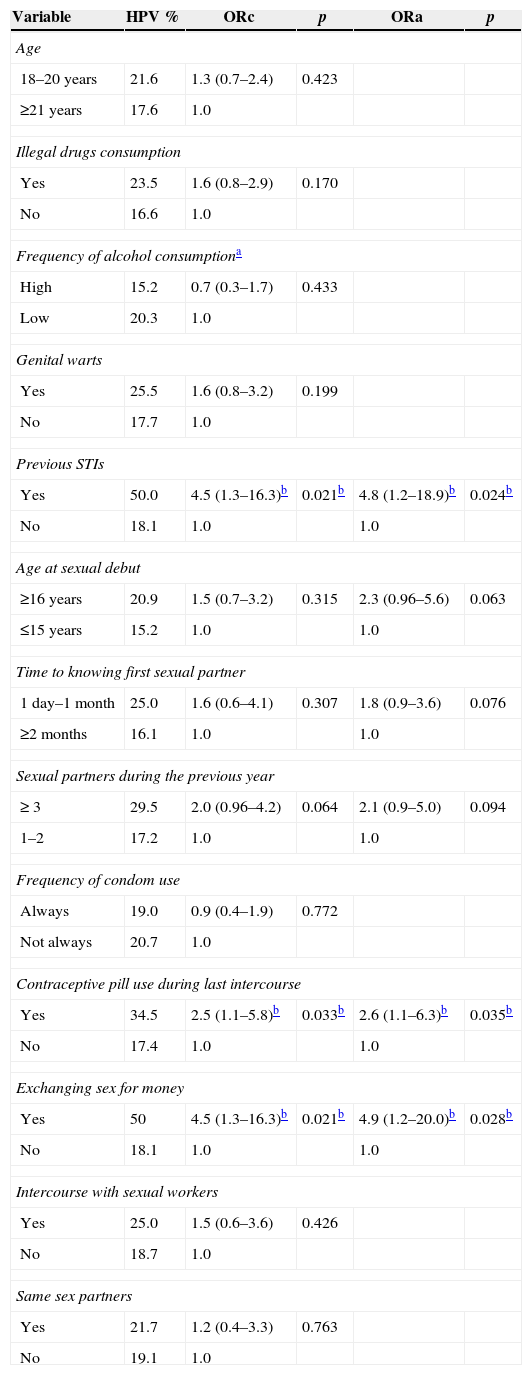
HPV prevalence was higher in students that consumed illegal drugs and in males with a history of genital warts, but without statistical significance. Nonetheless, half of the students with history of STIs presented HPV infection (p<0.05). Students with sexual debut at 16 years or older had 2.3-fold higher risk to HPV infection than students with sexual debut at 15 years or younger (ORa=2.3; 95% CI 0.96–5.6). Male students with later sexual debut and three or more sexual partners during last year, had 45.0% HPV infection and students with later sexual debut and two or less sexual partners during last year, had 18.0% HPV prevalence (p=0.005). The subjects who had had three or more sexual partners during last year had twice the risk of acquiring an HPV infection (ORa=2.1; 95% CI 0.9–5.0) than those with two sexual partners. Students who exchanged sex for money showed almost five times greater risk of having HPV, with statistical significance in multivariate analysis.
Male students, who had sex within one month after meeting a partner in comparison to those who reported a longer time to do so, had nearly twice the risk of becoming infected with HPV (ORa=1.8; 95% CI 0.9–3.6). This subgroup had higher rate of intercourse with sexual workers (14.1% vs. 6.8%, p=0.057), more casual sexual partners (47% vs. 32.9%, p=0.019) and a greater number of sexual partners (median 4 vs. 3, p=0.023, Mann Whitney). Finally, students whose partners had been using contraceptive pills during their last intercourse showed an HPV prevalence of 34.5%, i.e. a 2.6-fold higher risk than those students whose partners did not use contraceptive pills (p<0.05). Table 3 shows HPV prevalence according to risky sexual behaviors, and univariate and multivariate analyses.
Demographic and sexual behavior characteristics associated with HPV infection in Mexican male college students.
| Variable | HPV % | ORc | p | ORa | p |
|---|---|---|---|---|---|
| Age | |||||
| 18–20 years | 21.6 | 1.3 (0.7–2.4) | 0.423 | ||
| ≥21 years | 17.6 | 1.0 | |||
| Illegal drugs consumption | |||||
| Yes | 23.5 | 1.6 (0.8–2.9) | 0.170 | ||
| No | 16.6 | 1.0 | |||
| Frequency of alcohol consumptiona | |||||
| High | 15.2 | 0.7 (0.3–1.7) | 0.433 | ||
| Low | 20.3 | 1.0 | |||
| Genital warts | |||||
| Yes | 25.5 | 1.6 (0.8–3.2) | 0.199 | ||
| No | 17.7 | 1.0 | |||
| Previous STIs | |||||
| Yes | 50.0 | 4.5 (1.3–16.3)b | 0.021b | 4.8 (1.2–18.9)b | 0.024b |
| No | 18.1 | 1.0 | 1.0 | ||
| Age at sexual debut | |||||
| ≥16 years | 20.9 | 1.5 (0.7–3.2) | 0.315 | 2.3 (0.96–5.6) | 0.063 |
| ≤15 years | 15.2 | 1.0 | 1.0 | ||
| Time to knowing first sexual partner | |||||
| 1 day–1 month | 25.0 | 1.6 (0.6–4.1) | 0.307 | 1.8 (0.9–3.6) | 0.076 |
| ≥2 months | 16.1 | 1.0 | 1.0 | ||
| Sexual partners during the previous year | |||||
| ≥ 3 | 29.5 | 2.0 (0.96–4.2) | 0.064 | 2.1 (0.9–5.0) | 0.094 |
| 1–2 | 17.2 | 1.0 | 1.0 | ||
| Frequency of condom use | |||||
| Always | 19.0 | 0.9 (0.4–1.9) | 0.772 | ||
| Not always | 20.7 | 1.0 | |||
| Contraceptive pill use during last intercourse | |||||
| Yes | 34.5 | 2.5 (1.1–5.8)b | 0.033b | 2.6 (1.1–6.3)b | 0.035b |
| No | 17.4 | 1.0 | 1.0 | ||
| Exchanging sex for money | |||||
| Yes | 50 | 4.5 (1.3–16.3)b | 0.021b | 4.9 (1.2–20.0)b | 0.028b |
| No | 18.1 | 1.0 | 1.0 | ||
| Intercourse with sexual workers | |||||
| Yes | 25.0 | 1.5 (0.6–3.6) | 0.426 | ||
| No | 18.7 | 1.0 | |||
| Same sex partners | |||||
| Yes | 21.7 | 1.2 (0.4–3.3) | 0.763 | ||
| No | 19.1 | 1.0 | |||
ORc, crude odds ratio; ORa, adjusted odds ratio.
HPV prevalence among Mexican male college students analyzed stands at midpoint in comparison to other university populations: Japan3 and South Korea5 displayed an HPV prevalence of 1.3% and 10.6%, respectively, while students from Chile9 showed 83.6%. Studies from the US6–8 reported HPV prevalence between 25.8 and 42.8% in college students. However, the sampling sites and the diagnostic techniques were different; therefore the comparison between populations is difficult. HPV prevalence in Mexican male students analyzed was 19.4% and HPV16 was the prevailing detected type in the current study, similar to studies among young men in Kenya,25 Chile9 and the US.6 Further analysis taking into account genotypes revealed that HPV16 (single- or multiple-infection) displayed the highest viral load in comparison to other viral types. Higher viral load has been related to increased HPV transmission26 and higher HPV16 viral load has been associated with persistence of infection in women.27 Therefore, the male college students analyzed in our study are more likely to transmit HPV16 to their sexual partners.
It was noteworthy that a later sexual debut as considered in this paper (≥16 years) was associated with an HPV infection, in contrast to another study in which early sexual debut (≤17 years) was associated with HPV.5 In a previous study with the same population, we documented that HSV-2 antibodies were associated with a later sexual debut (≥18 years),28 because we found a subgroup of students with delayed sexual debut and a high turnover of sexual partners. Similarly, in the current analysis, male students with later sexual debut and higher turnover of sexual partners during last year had higher HPV infection (45%). Men whose partners were using contraceptive pills during their last relationship had more than twice the risk of acquiring HPV infection. The use of this contraceptive method is considered as an approximation of the lack of use of condoms. In a Canadian contraception study, only 27% of female contraceptive users reported concurrent use of condoms.29 Mexican male students who reported contraceptive pill use and no condom use (simultaneously) had 54.5% of HPV, in comparison to students taking contraceptive pills and using condom who had 22.2% of HPV prevalence (p=0.076). Moreover, Mexican female students from the same university who used the emergency contraceptive pill had twice the risk of acquiring HPV infection than non-users, because they had not used condoms.30 Male students with STIs history had almost five times the odds of getting HPV infection. Self-report of STIs could point to risky sexual practices,3 but in this sample of Mexican male students we have not detected any difference between students with and without self-report of STIs. This finding will need further assessment because the design of the study does not allow for a possible explanation. Exchanging sex for money and the number of sexual partners were the main risk factors for the acquisition of HPV infections, because both factors increase the likelihood of running into sexual partners with HPV infection. This association is consistent with other studies in young people.6,16,25,31
The current study does not represent all male students from the University, but could be indicative of the magnitude of HPV infection in that setting. Genital samples from young people are very difficult to obtain, but self-sampling allowed the HPV test to be performed. However, as not all college students participated, this could have generated a selection bias. We identified certain sexual behavior factors associated with HPV infection and we also showed that HPV16 was the most prevalent in the male college students analyzed and HPV16 was the genotype with the highest viral load. Longitudinal studies are needed to delve into the persistence and the elimination of the infection, to know more about HPV natural history in men, and to implement preventive measures in the student population, like condom promotion and vaccine use.
Conflicts of interestThe authors declare no conflicts of interest.
The study has been approved by the Institutional Review Board from the National Institute of Public Health from Mexico (code 88-6417), and we obtained written informed consent from all participants. The paper has been seen and approved by all the authors, the manuscript has not been simultaneously submitted or published elsewhere. This work was supported by the CONACyT, Grant: SALUD-2004-C01-34 and CVU was the recipient of a master degree scholarship by CONACyT.