To analyze the immunogenicity of virus-like particles (VLP) of human papillomavirus type 16 (HPV16) isolated in East China and the adjuvant potential of interleukin-12 (IL-12).
MethodsThe variant HPV16 L1VLP expressed in sf9 insect cells were purified with cesium chloride gradient centrifugation. BALB/c mice were vaccinated with VLP (L1N), VLP with Freund's adjuvant (L1A) or VLP with IL-12 recombinant plasmid (L1P). HPV16 VLP specific IgG and IFN-γ level in the serum were detected by ELISA, and the percentage of CD4+ and CD8+ in spleen cells was detected with flow cytometry.
ResultsThe titers of serum IgG antibodies in vaccinated groups were higher than in negative control and the serum antibodies mainly recognized conformation-dependent HPV16 VLP epitopes. Splenic CD4+ and CD8+ T cell subsets increased after vaccination in every experimental group, and CD8+ increased obviously in L1P group. The ratio of CD4+/CD8+ decreased in L1P group and increased in the other two groups, compared to control group. Vaccination induced specific secretion of IFN-γ in the serum of vaccinated group (p<0.05), especially in the L1P group.
ConclusionsVLP of HPV16 variant strain isolated in East China could induce humoral immunity and cellular immunity in mice, and IL-12 recombinant plasmid can enhance cellular immunity.
Cervical cancer is the fourth leading cause of female tumor mortality.1 It is estimated that 273,000 women die from cervical cancer every year worldwide. There is a close relationship between cervical cancer and human papillomavirus (HPV) infection, especially type 16.2,3 The use of prophylactic vaccine marks the great progress in preventing cervical cancer.4 Because of the region-specific HPV16 variant,5 it has not been verified that the current vaccine strain will be effective in all areas.
HPV capsid proteins have intrinsic capacity to self-assemble into virus-like particles (VLP) in different expression systems. Wild-type HPV16 VLP are used for constructing the current HPV vaccine because they not only elicit high titers of protective antibody and confer protection from experimental viral challenge, but also produce a cytotoxic T-cell response in mice or chimpanzees.6 In addition, a randomized double-blinded controlled trial demonstrated that the bivalent HPV16/18 L1 VLP vaccine purified from baculovirus-infected insect cells was generally safe, well tolerated and highly immunogenic.7
IL-12 is a multifunctional cytokine that plays an essential role in cell-mediated immunity. IL-12 not only inhibits the proliferation of tumor cells in animal models but also induces the abolition of virus infected-cells in some conditions.8 Therefore, IL-12 has great potential as a vaccine adjuvant for diseases such as virus infection and cancer, in which cellular immunity is crucially involved.
In this study, we investigated the immunogenicity of VLP of regional (East China) variant HPV16 L1 produced in sf9 insect cells infected by recombinant baculovirus, and examined the effects on cellular immunity of combined administration of IL-12 and VLP. The study shows that the current HPV16 variant VLP may be an ideal candidate vaccine for prevent and treat cervical cancer in East China.
Materials and methodsVirus strainIn this study we used HPV16 variant strain isolated from a cervical cancer patient in East China (GenBank accession no. AF393502).
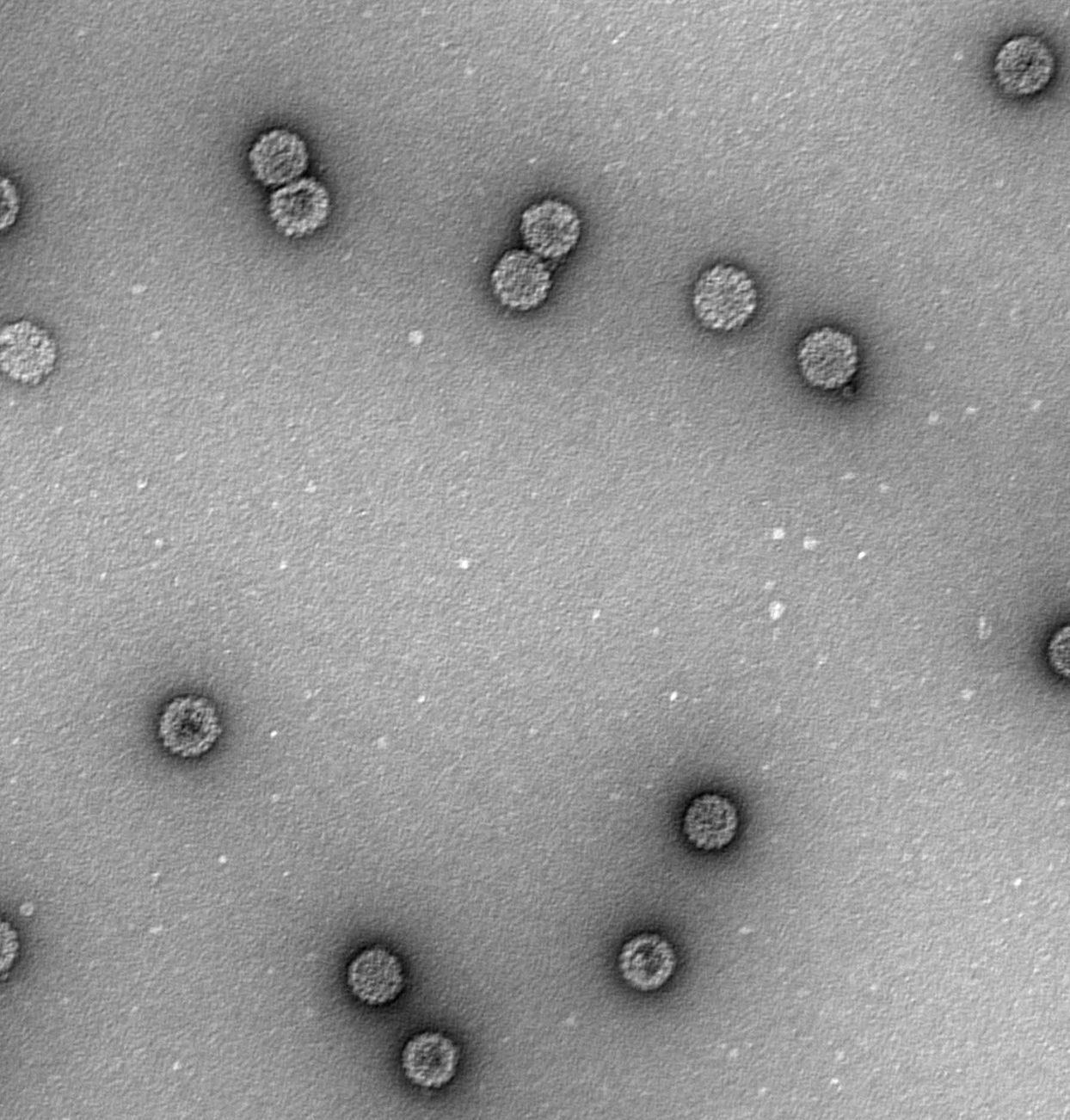
Purification of VLPFor the production of HPV16 L1 VLP, 1×109 sf9 cells were grown at 27°C in Grace's insect medium (Sigma) supplemented with 10% fetal calf serum, gentamycin and amphotericin. The cells were infected with recombinant baculovirus rBacV16 L1 at a MOI of 10. After 72h, the cells were harvested by low-speed centrifugation at 1000rpm (200×g) for 5min and washed once with ice-cold phosphate buffer saline (PBS). All subsequent procedures were carried out at 4°C. The cell pellet was resuspended in one volume of PBS to a total of 24mL and sonicated 30 times on ice at a setting of 200W. The total cell lysate was loaded onto a 40% (wt/vol) sucrose-PBS cushion and centrifuged at 25,000rpm (110,000×g) for 2.5h. The pellet was resuspended in 7mL of 35% (w/v) cesium chloride (CsCl)-PBS and centrifuged to equilibrium in 35% (wt/vol) CsCl-PBS for 20h at 35,000rpm (141,000×g). The visible band at a density of 1.29g/mL was harvested, dialyzed extensively against PBS, concentrated with PEG 2000, and VLP was examined with transmission electron microscopy (TEM) at 50,000 magnification. The prepared VLP was then stored at −80°C.
PlasmidThe plasmid pCAGGS-mIL-12 was prepared by standard alkaline lysis and DNA was further purified by banding CsCl2 gradient centrifugation as described previously.9
Immunization and sampling of miceSix to eight week-old female BALB/c mice were used in all experiments. The protocols for animal experiments were approved by the Ethics Committee of Harbin Medical University. The immunization protocols are shown in Table 1. Plasmids were injected intramuscularly, while VLP and Freund's adjuvant (Gibcol, BRL) were injected subcutaneously. Mice serum was obtained by retrobulbar collection two weeks after each immunization, when mice were sacrificed with decapitation methods. The spleen was dissected and the size was measured. Single-cell suspensions were obtained from the spleen by gently pressing the spleen between two sterile slides; dissociated cells were then washed in DMEM containing 5% fetal bovine serum (Sigma).
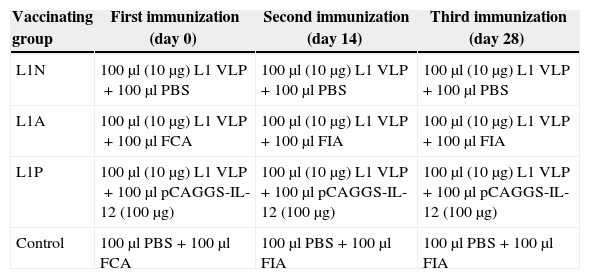
The immunization protocols.
| Vaccinating group | First immunization (day 0) | Second immunization (day 14) | Third immunization (day 28) |
|---|---|---|---|
| L1N | 100μl (10μg) L1 VLP+100μl PBS | 100μl (10μg) L1 VLP+100μl PBS | 100μl (10μg) L1 VLP+100μl PBS |
| L1A | 100μl (10μg) L1 VLP+100μl FCA | 100μl (10μg) L1 VLP+100μl FIA | 100μl (10μg) L1 VLP+100μl FIA |
| L1P | 100μl (10μg) L1 VLP+100μl pCAGGS-IL-12 (100μg) | 100μl (10μg) L1 VLP+100μl pCAGGS-IL-12 (100μg) | 100μl (10μg) L1 VLP+100μl pCAGGS-IL-12 (100μg) |
| Control | 100μl PBS+100μl FCA | 100μl PBS+100μl FIA | 100μl PBS+100μl FIA |
Splenocytes were incubated for 30min at 4°C with PE- or FITC-labeled CD4+, CD8+ monoclonal antibody (Zhongshan Biotech), followed by washing three times with PBS, then analyzed by fluorescence-activated cell sorter (FACS).
ELISA assayThe serum concentrations of interferon-γ (IFN-γ) were detected by using ELISA kit (Endogen) according to the manufacturer's instruction.
Detection of serum IgG antibodiesSerum IgG antibody titers were investigated using the HPV16VLP as the target antigen in a standard ELISA. Each well was coated with 100ng of purified VLP or denatured VLP (produced by the addition of 0.1M DTT and boiling) in 0.05M sodium carbonate buffer (pH 9.6) at 4°C overnight. Plates were then washed four times with TBS containing 0.1% Tween 20 (TBS/T). Non-specific binding sites in each well were blocked with 100μl of 1% BSA in TBS/T at 37°C for 1h. Then 100μl of serial 2-fold dilutions of serum were added to each well and incubated at 37°C for 1h. After washing, 100μl of 1:1000 HRP-conjugated goat anti-mouse IgG (Promega) were added and incubated at room temperature for 1h. Finally, the plates were developed by incubation with 100μl of OPD substrate reagent (2.43mL 0.1M citric acid, 2.57mL 0.2M Na2HPO4, 5mL H2O, 4mg OPD and 15μl 30% H2O2) for 5–15min in the dark, followed by quenching reaction with 100μl of 3M HCl. The absorbance was read at 490nm in an ELISA plate reader. Both HPV16 L1 monoclonal IgG CAMVIR-1 and HPV16 L1 polyclonal antibody M3 served as positive controls. CAMVIR-1 antibody was purchased from PharMingen. M3 antibody (kindly provided by Prof. Shang, Harbin Medical University) was collected from the serum after the mice were vaccinated with purified HPV16 L1 protein which was expressed in bacterial strains. All samples were examined in triplicate for each antibody class. The assay was considered valid only when the coefficient of variation of the triplicates was <10%. Sera with mean OD more than twice that of negative controls were considered positive for the respective IgG subclass.
Statistical analysisThe difference of splenic lymphocyte percentages and serum IFN-γ level were analyzed for statistical significance (p<0.05) using SigmaStat version 10.
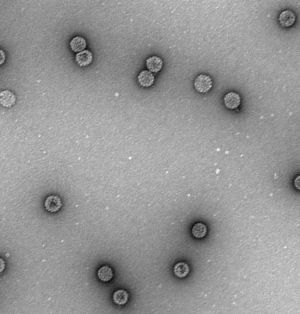
ResultsEfficient assembly of HPV16 L1 into VLPTo determine whether the variant HPV16 L1 protein could self-assemble efficiently, lysates from insect cells infected with L1 recombinant baculovirus rBacV16 L1 were subjected to CsCl2 density gradient centrifugation and the visible band was analyzed by TEM. The predominant structure consisted of spherical particles about 50nm in diameter with a regular array of capsomeres, although smaller, larger, and irregular spheres were also seen (Fig. 1). These results demonstrated that HPV16 L1 protein could efficiently self-assemble into VLP.
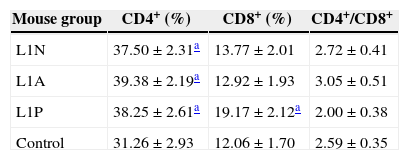
HPV16 L1 VLP and adjuvant immunity induce the proliferation of spleen T lymphocytesTo monitor the development of an immune response after the last immunization, spleen T lymphocytes were collected and subjected to FACS. The percentage of CD4+ T lymphocytes was increased in all three immunized groups compared with the control group. In addition, the percentage of CD8+ T lymphocytes was higher in L1P group than the other experimental groups and L1P group had the lowest CD4+/CD8+ ratio (Table 2).
CD4+, CD8+ splenic lymphocyte percentages and CD4+/CD8+ ratio in different groups after the last immunization.
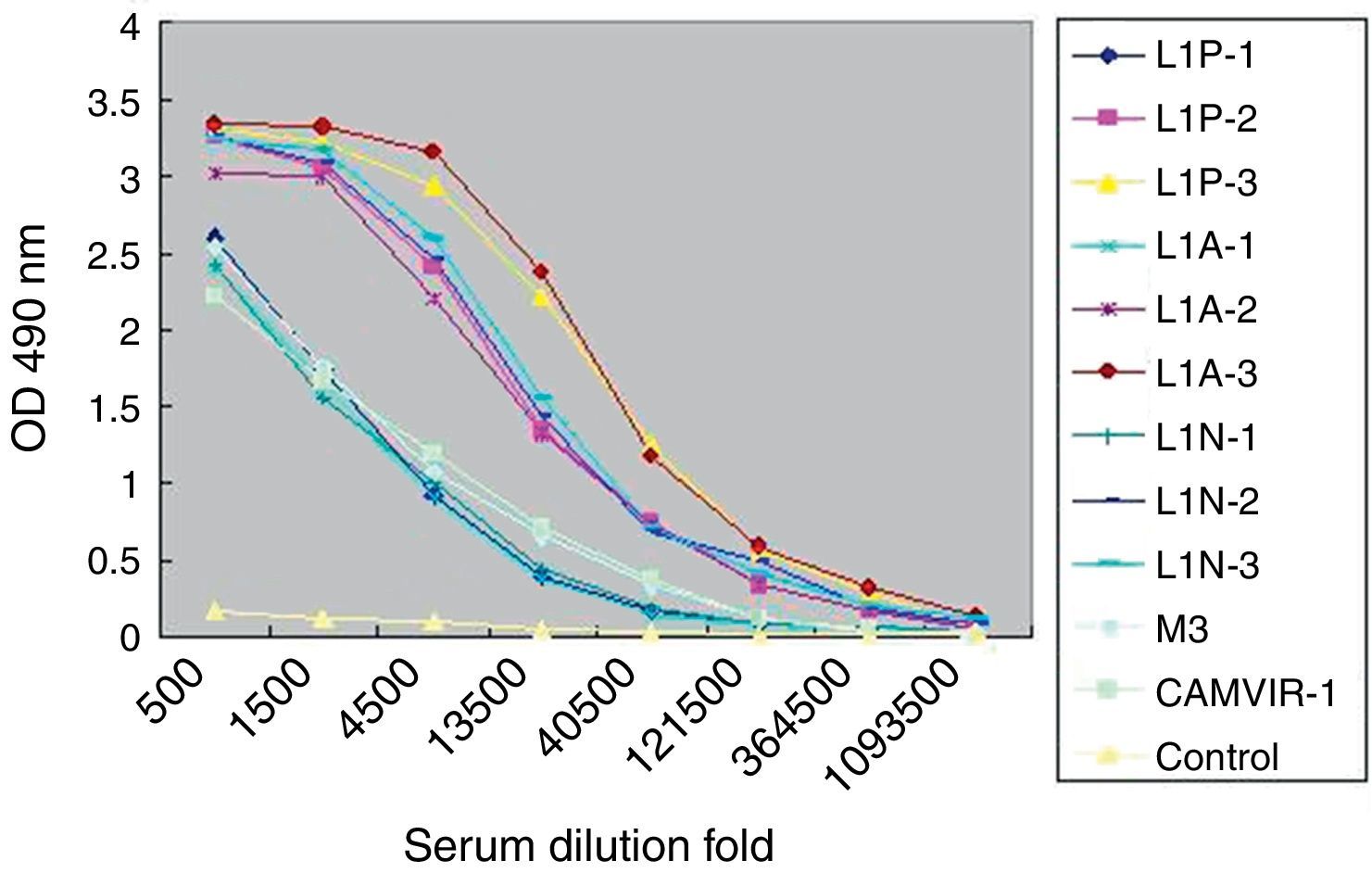
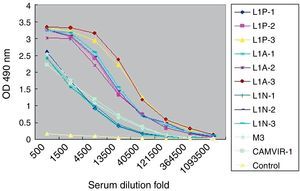
Purified HPV16 L1 VLP induced specific antibody in vaccinated mice (Fig. 2). When mice were given three consecutive booster doses, specific serum IgG responses to VLP16 increased. After the third immunization, IgG titers were 1:120,000 in L1N group and 1:360,000 in L1A and L1P groups. In contrast, by ELISA we determined that the titers of HPV16 L1 monoclonal IgG CAMVIR-1 (PharMingen) and polyclonal antibody M3 to purified VLP were only 1:13,500. No specific IgG was detected in control group.
Titers of serum IgG antibodies from vaccinated mice. L1N, L1A, L1P, control indicated sera from the corresponding group of mice. -1, -2, -3 indicated the first, second and third immunization. M3 indicated sera antibodies from mice immunized with HPV16 L1 expressed in Escherichia coli. CAMVIR-1 indicated commercial HPV16 L1 monoclonal antibody.
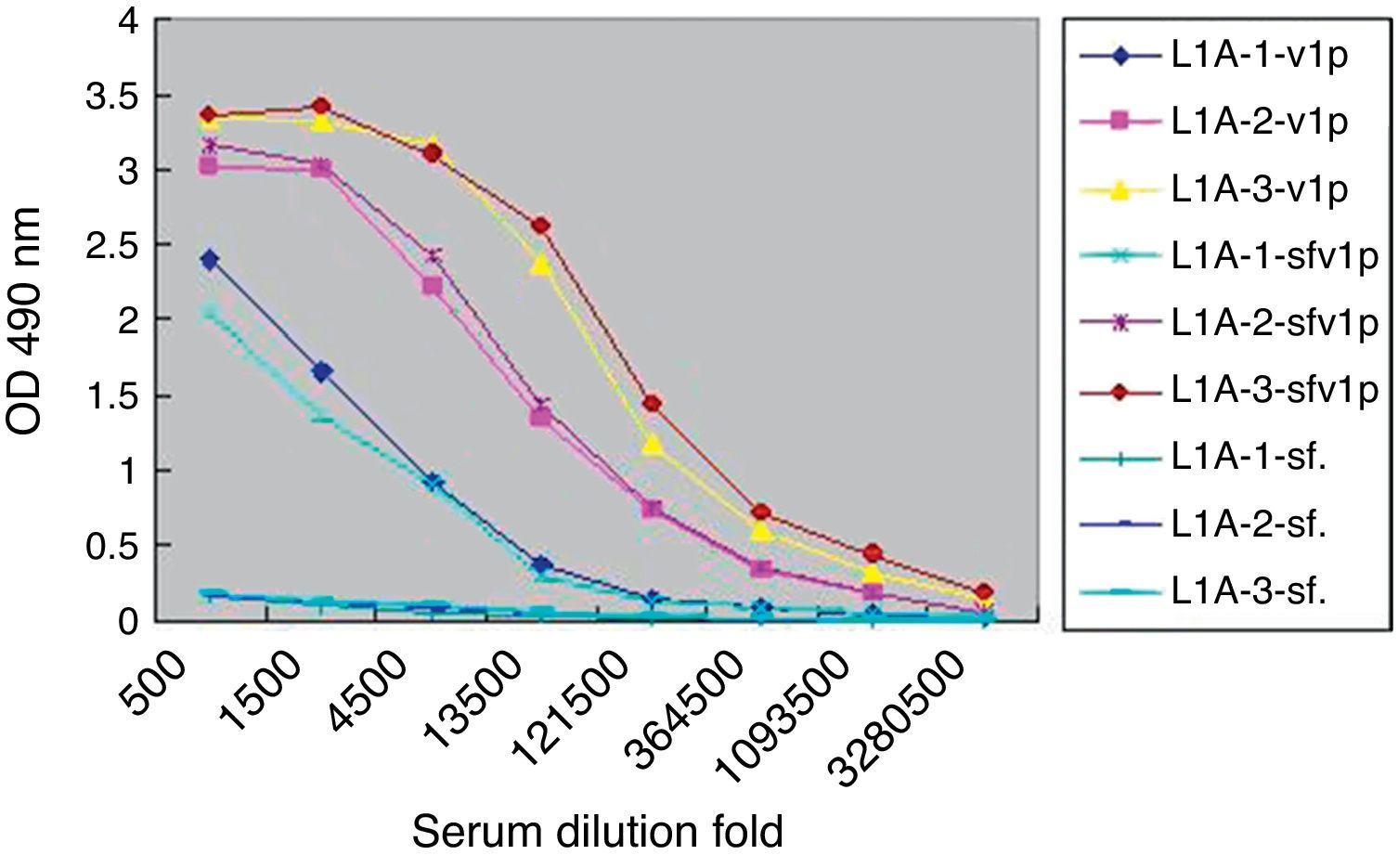
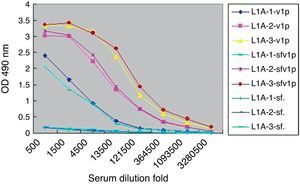
Considering the intrinsic capacity of HPV L1 to self-assemble into VLP in sf9 cells, the lysates of sf9 infected with rBacV16 L1 were used as VLP antigen to analyze the specific IgG produced in L1A group (Fig. 3). ELISA results showed that the titers of specific IgG to the lysates of infected sf9 cells were the same as purified VLP. No positive results were found for the lysates of normal sf9 cells. Similar results were observed in the other two immunized groups.
Titers of sera IgG antibodies to purified VLP and lysates of infected Sf9 cell. L1A indicated sera from the L1A group of mice. -VLP indicated ELISA plate coated with VLP. -sfvlp indicated ELISA plate coated with lysates of Sf9 cells infected by rBacV16 L1. -sf. indicated ELISA plate coated with lysates of normal Sf9 cells.
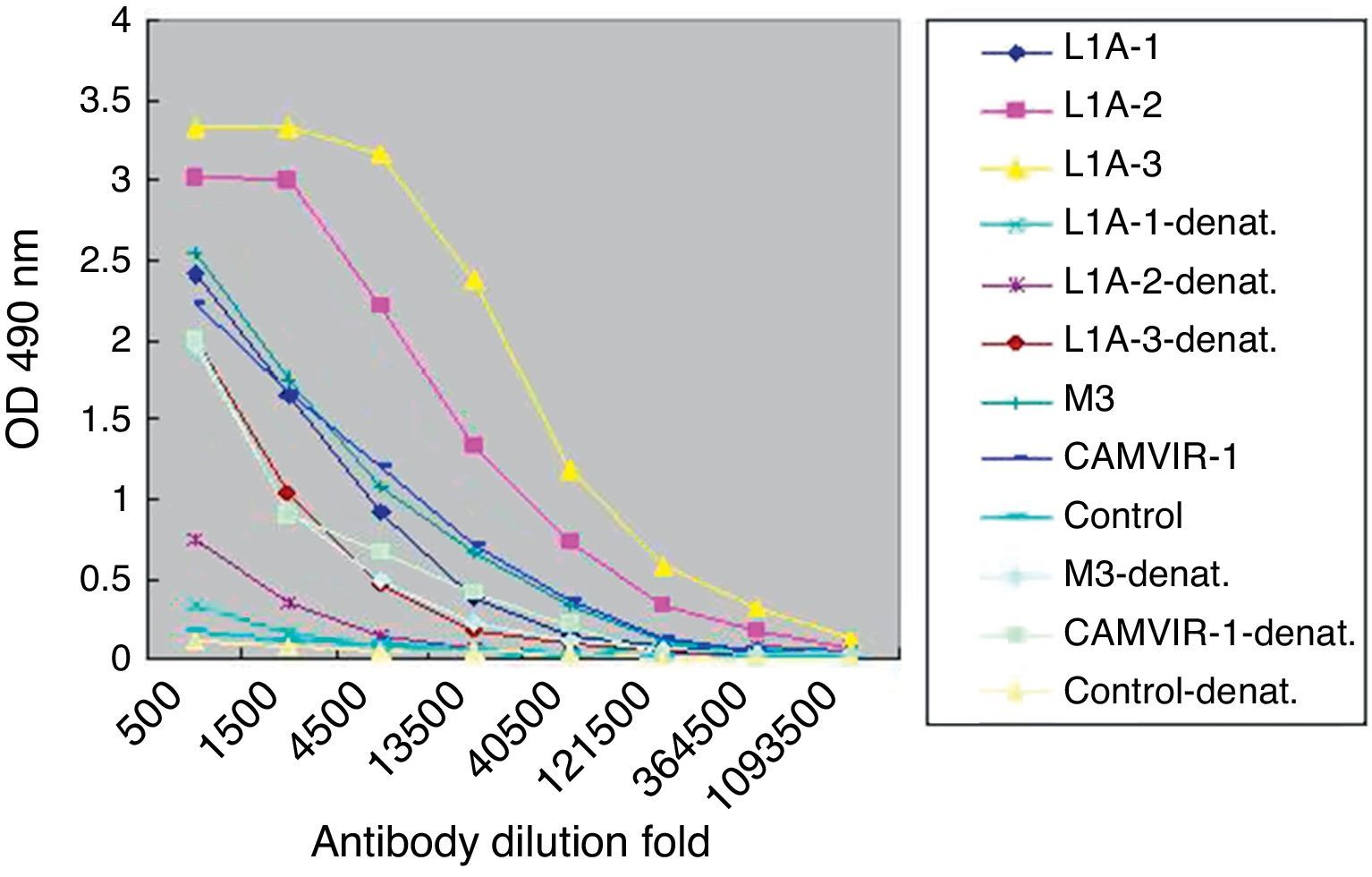
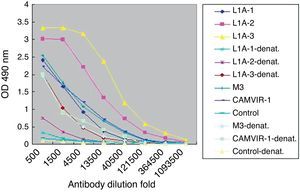
Next we used denatured VLP to develop ELISA for L1A group serum (Fig. 4). Compared to the high titers to the purified VLP (1:360,000), the titers of specific IgG to denatured VLP (1:13,500) were significantly decreased. Similar results were observed in the other two immunized groups.
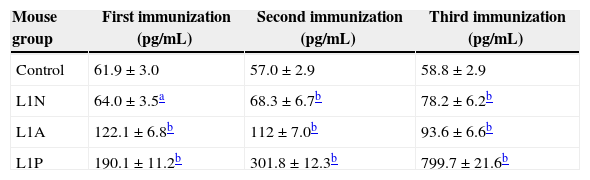
IL-12 promotes cellular immunity responses stimulated by VLP in immunized miceSerum from immunized mice was examined for the concentration of Th1-type cytokine IFN-γ (Table 3). Only a slight increase in serum IFN-γ level was observed in L1N group after three boosts, but serum IFN-γ level was significantly boosted after three consecutive immunizations in L1P group (799.7pg/mL). In L1A group, after the first immunization serum IFN-γ level increased to 190pg/mL, but then decreased slowly with continued immunization. Serum IFN-γ level in every vaccination group was higher than that in control mice (p<0.05).
Serum IFN-γ level in mice vaccinated with HPV16 L1 VLP.
Numerous HPV16 variants have been isolated from different geographic regions,5,10,11 but current HPV16 preventive vaccine focused on a European strain.12 In the present study, we used HPV16 variant strain isolated from a cervical cancer patient in East China, which was grouped into Asian-American type. In this study, we produced VLP of a regional variant HPV16 L1, and then immunized three groups of BALB/c mice with VLP (L1N), VLP with Freund's adjuvant (L1A) or VLP with recombinant IL-12 plasmid (L1P), respectively.
ELISA assay showed that specific IgG against natural VLP was produced after immunization. Combination with Freund's adjuvant (L1A group) or recombinant IL-12 plasmid (L1P group) increased IgG titers, compared to stimulation with VLP alone (L1N group). In either L1A or L1P group the antibody titers increased with the boost interval elongated. For denatured VLP, IgG titers decreased significantly compared to nature VLP. Notably, the titers of HPV16 L1 monoclonal IgGCAMVIR-1 and polyclonal antibody M3 were the same for nature or denatured VLP, suggesting that these antibodies recognize linear epitopes.
Although VLP-induced neutralizing antibodies appear sufficient for the protection from experimental challenge with HPV, cellular immune responses are likely to play an important role in viral clearance.13 Transient induction of HPV specific CTL and frequent release of IFN-γ were detected in HPV16 VLP immunized chimpanzees.14 In this study, both the ratio of CD4/CD8+ splenic lymphocytes and serum IFN-γ level increased in every immunized group. Enhanced IFN-γ secretion indicates the transformation of T lymphocytes from Th0 to Th1. CD8+ T lymphocytes are killer cells and the increased ratio of CD4/CD8+ indicates the stimulation of cellular immune response. In the L1A group serum IFN-γ level was higher at the first boost than the later boosts. We mixed Freund's complete adjuvant (FCA) with VLP in the first boost and Freund's incomplete adjuvant (FIA) in the second and third boosts. It is thought that FIA stimulated humoral immunity reaction, while FCA helped both humoral and cellular immunity reaction.
Our results showed that the variant HPV16 L1 VLP alone could induce both mucosal and cellular immune responses, suggesting that the VLP has the potential for being a therapeutic vaccine. IL-12 is an important antitumor and antivirus cytokine. IL-12 has been shown to be an effective adjuvant in several systems.15 Here we showed that vaccinated DNA encoding IL-12 with VLP increased cellular immunity reaction. Purified recombinant IL-12 protein as adjuvant can stimulate cellular immunity. However, due to its short life-time a large dose of IL-12 protein is required and could increase the toxicity of IL-12. Naked DNA injection has been developed successfully for the evaluation of the antitumor activity of IL-12 in animal therapy models.16–18 In this study the mice in L1P group were injected with VLP and IL-12 recombinant plasmid, and they stimulated cellular reaction efficiently. The percentage of CD8+ increased obviously, the ratio of CD4+/CD8+ decreased and serum IFN-γ level increased greatly. The titers of specific IgG antibodies in L1P and L1A groups were similar, but there were subtype difference. IL-12 immunity was associated with increased IgG2α but not IgG1. In addition, we found that the spleen was 2–3 times bigger in L1P group than in the other groups, perhaps due to IL-12 induced proliferation of splenic cells.
VLP can self-reassemble after disassembling to L1 components in the presence of reducing agents.19 Previous studies demonstrated that HPV16 VLP self-assembled from L1 protein could encapsidate heterologous DNA up to 8.0kb in vitro, and the pseudovirion can infect many kinds of cell lines.20,21 If IL-12 expressing plasmid DNA could be encapsidated in VLP, this pseudovirion could be an ideal biochemical agent to be used as a therapeutic agent for HPV infection and cervical cancer.CD4+
In summary, our study demonstrates that VLP of HPV16 variant strain isolated in East China could induce humoral immunity and cellular immunity in mice, and IL-12 recombinant plasmid can enhance cellular immunity.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Prof. Sakai T and Dr. Qinglong Shang for the generous donation of pCAGGS-mIL-12 and polyclonal antibody M3. This study was supported by grants from the National Natural Science Foundation of China (Nos. 30901706, 81101235 and 81000726), the Natural Science Foundation of Heilongjiang Province (No. ZD201020), the Scientific Research Foundation for ROCS, SEM (No. 2011-508) and the National Key Basic Research Program of MOST (No. 2012CB526705). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.