Human immunodeficiency virus (HIV)-associated neurological complications continue to occur despite the development in antiretroviral treatment. New forms of old opportunistic infections and increased prevalence of neurocognitive disorders are the challenges that infectious diseases specialists face in daily clinic. How to screen and treat these disorders are subject of debate and new studies are underway to answer these questions. This review focuses on a brief discussion about opportunistic infections still present in late diagnosed HIV-infected patients and describes new forms of HIV-related neurological complications.
The central nervous system (CNS) is a common site of involvement in HIV patients. The spectrum of neurological complications of HIV is very broad, including complications caused by HIV itself as well as complications due to opportunistic pathogens and neoplasms.1
Following the introduction of highly active antiretroviral therapy (HAART), the prevalence of opportunistic infections (OIs) decreased in many areas of the world. However, in developed countries patients continue to present with OIs as the initial manifestation of HIV infection because of late diagnosis of HIV, non-adherence to antiretroviral or prophylactic treatments or unavailability of therapeutic and preventive options.
Moreover, syndromes such as HIV-associated neurocognitive disorder (HAND) have become more prevalent, according to some studies.2 New forms were described, and more often infectious diseases specialists face the challenge to investigate patients with few, if any, neurological complaints, even in the presence of suppressed viral load.
This review focuses on recent developments in the field of neurological complications of HIV in the HAART era, and will discuss old OIs and the new forms of HIV-related neurological complications.
The “old OIs”The most common intra-cranial lesions in acquired immunodeficiency syndrome (AIDS) patients are toxoplasma encephalitis (TE), cryptococcal meningitis with mucinous pseudocysts formation (CM), primary central nervous system lymphoma (PCNSL), and progressive multifocal leukoencephalopathy (PML).3 In developing countries such as Brazil, neurotuberculosis is an important differential diagnosis for TE, being the second most common HIV intracranial complication.4Table 1 shows the main MRI patterns of the most common etiologies of CNS involvement in HIV-infected patients.
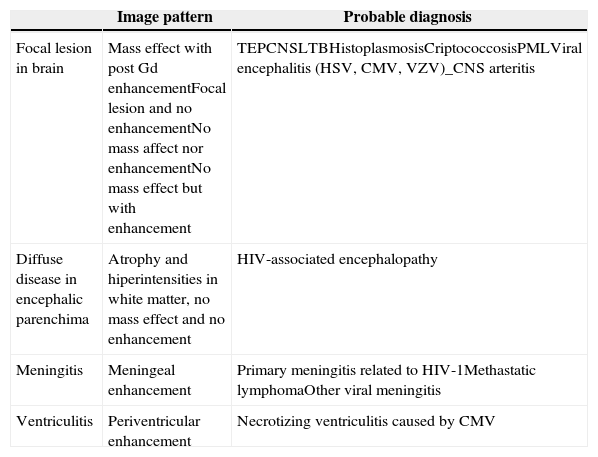
Common image patterns in MRI related to neurological complications of HIV and main etiologies.
| Image pattern | Probable diagnosis | |
|---|---|---|
| Focal lesion in brain | Mass effect with post Gd enhancementFocal lesion and no enhancementNo mass affect nor enhancementNo mass effect but with enhancement | TEPCNSLTBHistoplasmosisCriptococcosisPMLViral encephalitis (HSV, CMV, VZV)_CNS arteritis |
| Diffuse disease in encephalic parenchima | Atrophy and hiperintensities in white matter, no mass effect and no enhancement | HIV-associated encephalopathy |
| Meningitis | Meningeal enhancement | Primary meningitis related to HIV-1Methastatic lymphomaOther viral meningitis |
| Ventriculitis | Periventricular enhancement | Necrotizing ventriculitis caused by CMV |
TE, toxoplasma encephalitis; PCNSL, primary central nervous system lymphoma; TB, tuberculosis; PML, progressive multifocal leukoencephalopathy; HSV, herpes simplex virus; CMV, cytomegalovirus; VZV, varicella-zoster virus; CNS, central nervous system.
TE is the most common cause of CNS mass lesions in patients infected with HIV. The seroprevalence of toxoplasma varies from 10% to 40% in the United States to 70% to 90% in Europe. Toxoplasma gondii (TG) is a protozoan which can also cause disease in immunocompetent patients, but in this setting, the disease is usually benign and does not affect the CNS.5,6
Usually, the CD4 cell count of patients with HIV presenting with TE is less than 100, but some patients can develop the disease with CD4 cell counts between 100 and 200 cells/mm3.7 The prevalence of TE has decreased with the use of trimethoprim sulfamethoxazole for prophylaxis against Pneumocystis jirovecii.8Table 2 shows the diagnosis and treatment characteristics of CNS OIs.
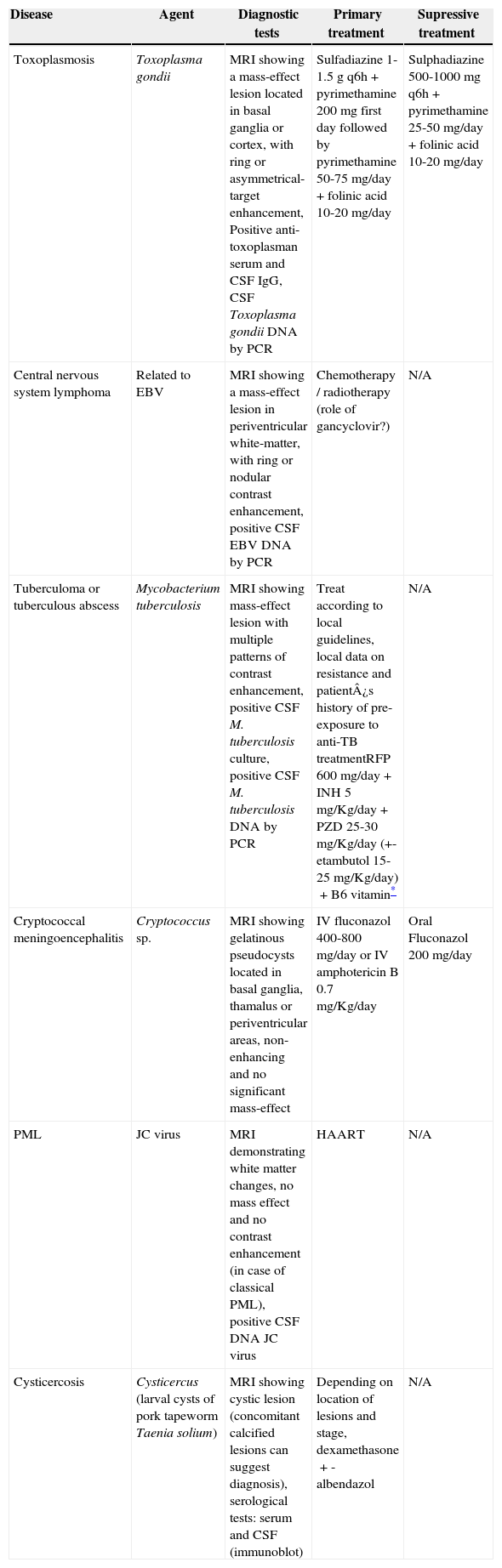
Intra-cranial diseases, their etiologic agents, useful tests for diagnosis and treatment options.
| Disease | Agent | Diagnostic tests | Primary treatment | Supressive treatment |
|---|---|---|---|---|
| Toxoplasmosis | Toxoplasma gondii | MRI showing a mass-effect lesion located in basal ganglia or cortex, with ring or asymmetrical-target enhancement, Positive anti-toxoplasman serum and CSF IgG, CSF Toxoplasma gondii DNA by PCR | Sulfadiazine 1-1.5g q6h+pyrimethamine 200 mg first day followed by pyrimethamine 50-75 mg/day+folinic acid 10-20 mg/day | Sulphadiazine 500-1000mg q6h+pyrimethamine 25-50 mg/day+folinic acid 10-20 mg/day |
| Central nervous system lymphoma | Related to EBV | MRI showing a mass-effect lesion in periventricular white-matter, with ring or nodular contrast enhancement, positive CSF EBV DNA by PCR | Chemotherapy / radiotherapy (role of gancyclovir?) | N/A |
| Tuberculoma or tuberculous abscess | Mycobacterium tuberculosis | MRI showing mass-effect lesion with multiple patterns of contrast enhancement, positive CSF M. tuberculosis culture, positive CSF M. tuberculosis DNA by PCR | Treat according to local guidelines, local data on resistance and patient¿s history of pre-exposure to anti-TB treatmentRFP 600 mg/day+INH 5 mg/Kg/day+PZD 25-30 mg/Kg/day (+- etambutol 15-25 mg/Kg/day)+B6 vitamin* | N/A |
| Cryptococcal meningoencephalitis | Cryptococcus sp. | MRI showing gelatinous pseudocysts located in basal ganglia, thamalus or periventricular areas, non-enhancing and no significant mass-effect | IV fluconazol 400-800 mg/day or IV amphotericin B 0.7 mg/Kg/day | Oral Fluconazol 200 mg/day |
| PML | JC virus | MRI demonstrating white matter changes, no mass effect and no contrast enhancement (in case of classical PML), positive CSF DNA JC virus | HAART | N/A |
| Cysticercosis | Cysticercus (larval cysts of pork tapeworm Taenia solium) | MRI showing cystic lesion (concomitant calcified lesions can suggest diagnosis), serological tests: serum and CSF (immunoblot) | Depending on location of lesions and stage, dexamethasone+- albendazol | N/A |
MRI, magnetic resonance imaging; CSF, cerebrospinal fluid; PCR, polymerase chain reaction; EBV, Epstein-Barr virus; N/A, not available; TB, tuberculosis; RFP, rifampin; INH, isoniazid; PZD, pyrazinamide; PML, progressive multifocal leukoencephalopathy; HAART, highly active antiretroviral therapy.
Clinical characteristics of TE are nonspecific and depend on where the lesion is located in the brain.6 Most commonly, lesions from TE are located in the basal ganglia or subcortical white matter, although any part of the CNS can be involved. Concomitant diffuse atrophy disproportionate to the patient's age is very common, present in more than 1/3 of patients. TE lesions are usually abscesses of more than 3cm in diameter that most commonly show enhancement after contrast injection. On brain CT, lesions are hypodense and surrounded by edema and enhance with contrast, classically presenting “ring enhancement” or “asymmetric target sign”. MRI can often reveal multiple lesions in a patient presenting with a single lesion on CT.5
The diagnosis of TE is based on clinical and laboratory findings. A high degree of suspicion is warranted for a patient with HIV infection, CD4 less than 100 cells/mm3, positive serum anti-toxoplasma IgG (which demonstrates previous contact with the protozoan), and focal signs on neurological exam. Brain CT or MRI should be performed with pre- and post-contrast images, but in many resource-limited settings these may not be available. If the diagnosis is suspected, an empiric treatment with sulphadiazine, pyrimetamine, and folinic acid should be instituted.6
Usually, TE shows a rapid response to therapy as shown by image resolution or clinical improvement. Treatment is considered a failure if no clinical and radiographic improvements are observed after two weeks of therapy. In such cases, a brain biopsy is indicated to search for an alternative diagnosis.6
The principal differential diagnosis of TE in some countries is PCNSL.9 This is a neoplasm linked to infection with Epstein-Barr virus (EBV) and occurs in advanced HIV disease (CD4 less than 100 cells/mm3). It causes mass lesions with multiple patterns of contrast enhancement on radiographic imaging, very similar to TE. Diagnosis can be made by CSF analysis with presence of neoplastic cells on flow cytometry, associated with a positive EBV DNA by polymerase chain reaction (PCR). Chemotherapy and radiotherapy are the treatment.3
Leptomeningitis is very common in the clinical picture of CNS TB. Also, CNS TB can cause infarction, abscess formation, and granuloma.10 Usually, the lesions present with mass-effect and IV contrast enhancement on CT and MRI.11 CSF tests can help to make the diagnosis, with a mononuclear pleocytosis, low glucose, and increased protein levels. CSF must be sent for M. tuberculosis culture, but this can take weeks to produce a result. M. tuberculosis DNA by PCR in CSF may lead to a faster diagnosis, but the sensitivity is variable. Additionally, a search for extra-neural sites of tuberculosis can be helpful. Around 20% of patients will present with extra-neural signs of active tuberculosis, such as lung lesions, that are more easily accessible for collection of material to confirm the diagnosis.10
Cryptococcus neoformans, besides being a very common cause of meningitis, can also cause gelatinous pseudocyst formation, with the deposit of a thick material in the perivascular spaces of periventricular white-matter (Virchow-Robin spaces). Usually, these do not enhance after IV contrast injection, and they cause no mass effect. Although not pathognomonic, their appearance is highly suggestive of cryptococcosis.12
Neurocysticercosis is among the most prevalent CNS infections in many developing countries. It is diagnosed when a patient in endemic areas shows a radiographic image compatible with the disease (i.e. a cystic lesion with different patterns of enhancement after IV contrast) and has a positive CSF serologic reaction (immunoblot).13
The prevalence of neurocysticercosis is not higher among HIV-infected individuals.14 However, some atypical cases have been described, mostly in resource-limited settings. Treatment should be started with corticosteroids and/or cysticidal therapy (praziquantel or albendazol), according to the stage and topography of the disease in the CNS.
What is new with the old CNS OIs?Progressive multifocal leukoencephalopathy (PML) is a disease caused by the polyomavirus JC virus (JCV), and is associated with severe immunosupression (CD4 less than 100 cells/mm3), although some cases with a higher CD4 count have been described. The main feature is a progressive lesion of white matter causing variable neurologic signs and symptoms, no fever, and a progressive disease. Diagnosis is suggested by the typical appearance in MRI complemented by a positive CSF JCV DNA by nested PCR. There is no specific treatment, but improvement or at least stability has been reported in some patients due to HAART.15
Koralnik et al. described an HIV-infected patient with JCV infection restricted to granule cell neurons of the cerebellum and with compatible symptoms. That was a description of a new syndrome called JCV granule cell neuronopathy. Patients with this new variant have no typical white matter changes on MRI but present with cerebellar atrophy.16
Another variant of the “classical” PML is the “inflammatory” PML. Patients with previous diagnosis of PML who start HAART develop worsening of previous known PML.17 In some cases, patients have undiscovered subclinical PML during HAART.18 In both cases, image tests are different from classical PML because in inflammatory PML, an MRI can show mass effect and enhancement. This entity was reported to be associated with fatal cases of PML. Steroids may help to reduce the exacerbated inflammatory response and contribute to better outcomes.
HIV-associated neurocognitive disorderHIV-associated neurocognitive disorder (HAND) is the new definition for what used to be called AIDS-dementia complex – the phenotypic form of HIV encephalitis. In the 1993 Centers for Disease Control and Prevention (CDC) classification, HAND was defined as an AIDS-defining illness.
HAND is the most common cause of dementia in young people, and one of the few treatable dementias. It usually has an insidious onset and a chronic, progressive course, bringing disability to many HIV patients. Also, this syndrome may cause poor adherence to HIV care, job loss, decline of driving ability, and lesser adherence with HIV medications, with increased morbidity and mortality.
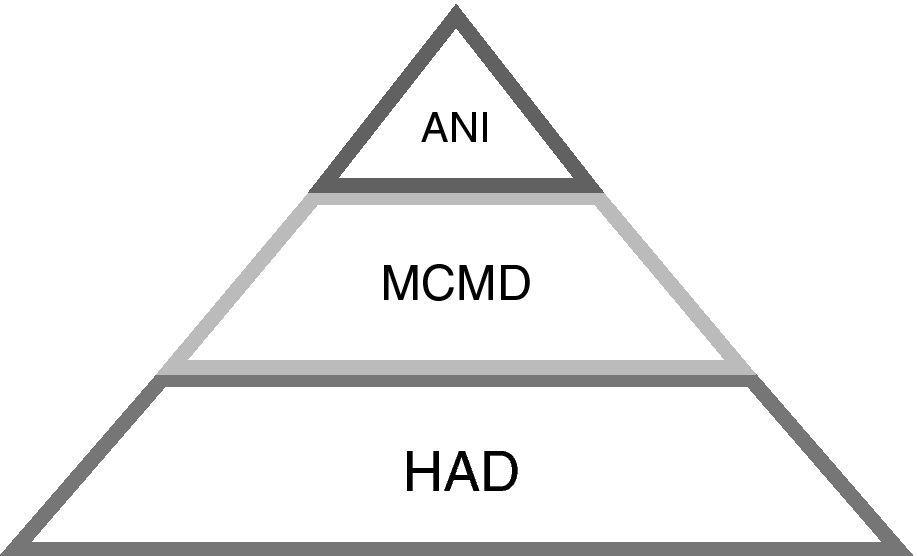
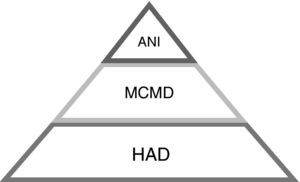
In 2007, new criteria were published defining how to diagnose HAND, including a wide spectrum of HIV-related cognitive impairments19 (Table 3, Fig. 1) as follows:
- •
Asymptomatic neurocognitive impairment (ANI)
- •
Mild cognitive motor disorder (MCMD)
- •
HIV-associated dementia (HAD).
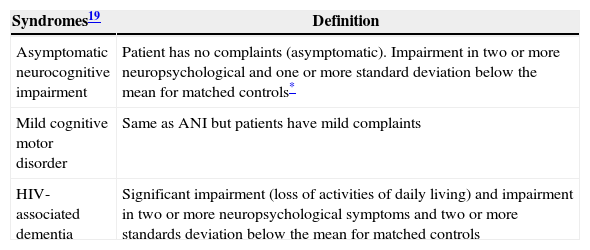
HAND definitions.
| Syndromes19 | Definition |
|---|---|
| Asymptomatic neurocognitive impairment | Patient has no complaints (asymptomatic). Impairment in two or more neuropsychological and one or more standard deviation below the mean for matched controls* |
| Mild cognitive motor disorder | Same as ANI but patients have mild complaints |
| HIV-associated dementia | Significant impairment (loss of activities of daily living) and impairment in two or more neuropsychological symptoms and two or more standards deviation below the mean for matched controls |
ANI, asymptomatic neurocognitive impairment.
In the HAART era, the incidence of HAND has decreased, but the prevalence has paradoxically increased, with mild forms of the disease observed more commonly than before. In developing countries, patients often died before the diagnosis of HAND could be made. However, with the broad use of HAART, this scenario is changing.
Although HAND is observed more frequently in patients with severe immunosupression (usually a CD4 count less than 100 cells/mm3), it can occur in patients with higher CD4 cell counts, especially in its subtle forms. CD4 nadir less than 50 cels/mm3 is a risk factor for development of neurocognitive disorder in HIV patients.20
HAND is characterized by the triad of cognitive impairment, behavioral abnormality, and motor dysfunction. Classically, HIV dementia is considered a subcortical dementia, characterized by more motor symptoms and less memory and language impairment than is commonly seen in cortical dementias such as Alzheimer's disease.21 Recently, asymptomatic and mild forms of neurocognitive impairment were shown to actually increase the risk for future symptomatic decline. This may be a reason why infectious diseases specialists should actively screen HIV patients with no neurological or cognitive complains in daily practice.22
The clinical diagnosis of HAND is ideally based on the patient's performance on neurocognitive tests, as described. Unfortunately these tests need to be applied by specially trained professionals (such as psychologists or neurologists), and in many resource-limited countries they are not widely available. Another way to screen patients for neurocognitive impairment is through the use of scales, such as the HIV Dementia Scale or the International HIV Dementia Scale. The latter is a bedside test that has been developed and applied in populations from the United States and Uganda, showing high specificity and sensitivity. This scale was also useful when applied to HIV-infected patients from India. This test can be administered by non-neurologists, and is a useful tool for detection of cognitive impairment. If a patient screens positive by this scale, he/she can be referred for further neurocognitive evaluation with additional neuropsychological testing.23
CSF findings in HAND patients are non-specific, usually showing normal or mild pleocytosis, normal or mildy increased protein and normal glucose. It is important to exclude opportunistic infections. CSF HIV-1 viral load is not reliable in the diagnosis of cognitive disorder caused by HIV infection itself but, when positive, it may suggest a CSF HIV escape and may guide therapy to a regimen with improved CSF penetration, according to penetration score.24
Cranial CT and MRI may show cortical atrophy, but the degree of atrophy has not been correlated with the grade of dementia. MRI may show diffuse white-matter hyperintensities in the deep white matter with atrophy of bilateral caudate nucleus, but these results are non-specific. Care should be taken with the use of imaging studies, because patients with HAND may have a normal MRI and patients with bilateral white matter hyperintensities may have an alternative diagnosis. Image studies must rule out alternative diagnosis.
There is no specific treatment for HAND. Antiretroviral drugs that penetrate the blood brain barrier (BBB) are currently considered the best choices for treatment of these patients.25,26
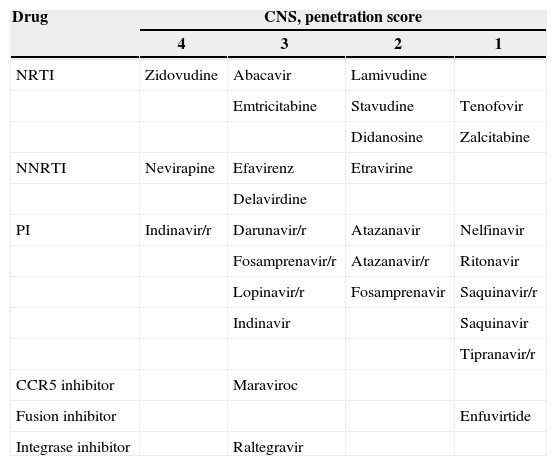
Penetration of ARV through the BBB: the CPE scoreMany drugs were investigated in the treatment of HAND with no good results so far. At this moment, the best treatment for HAND is ARV with good CNS penetration. In order to evaluate general characteristics of available ARV to penetrate the CNS, Letendre et al. developed a CNS penetration score (CPE) (Table 4), which showed that higher scores were related to lower CSF viral load.25,26
CNS penetration score.
| Drug | CNS, penetration score | |||
|---|---|---|---|---|
| 4 | 3 | 2 | 1 | |
| NRTI | Zidovudine | Abacavir | Lamivudine | |
| Emtricitabine | Stavudine | Tenofovir | ||
| Didanosine | Zalcitabine | |||
| NNRTI | Nevirapine | Efavirenz | Etravirine | |
| Delavirdine | ||||
| PI | Indinavir/r | Darunavir/r | Atazanavir | Nelfinavir |
| Fosamprenavir/r | Atazanavir/r | Ritonavir | ||
| Lopinavir/r | Fosamprenavir | Saquinavir/r | ||
| Indinavir | Saquinavir | |||
| Tipranavir/r | ||||
| CCR5 inhibitor | Maraviroc | |||
| Fusion inhibitor | Enfuvirtide | |||
| Integrase inhibitor | Raltegravir | |||
CNS, central nervous system; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor.
Other studies presented discordant results when analyzing neuro-HAART (HAART constituted by drugs with improved BBB penetration) ability to improve neurocognitive impairment, with one study showing worsening of cognitive performance in patients with higher CPE scores.27 This result questioned neural toxicity but, overall, more randomized clinical trials are needed to better answer the question of whether neuro-HAART can really improve cognitive performance in HAND patients.
At this moment, starting neuro-HAART in patients with no cognitive impairment is not indicated, but some guidelines already suggest changing the ARV regimen in patients diagnosed with HAND in order to improve their cognitive performance.28
In conclusion, neurological involvement in HIV-infected patients is still important, even in the post-HAART era, with not only new forms or atypical presentations of old OIs, but also milder forms of neurocognitive impairment. New clinical trials investigating easier diagnostic tools for patients with cognitive impairment, the utility of neuro-HAART, and clinical evaluation of those patients are ongoing to answer many doubts the scientific community still has.
Conflict of interestThe author declares to have no conflict of interest.