Cysteine proteinases are well-known virulence factors of Leishmania spp. with demonstrated actions in both experimental mouse infection and human infection. However, studies on these enzymes in canine leishmaniasis are scarce. Here, we show, for the first time, the reactivity of sera from dogs living in an endemic area to a recombinant protein from the COOH-terminal region of cysteine B protease. In this work, enzyme-linked immunosorbent assays were performed using a 14kDa rcyspep protein obtained through a pET28-a expression system in Escherichia coli. First, 96-well plates were coated with rcyspep (500ng/well) and incubated with sera from dogs (1:100). Subsequently, IgG antibody detection was performed using rabbit anti-dog IgG antibodies conjugated with peroxidase. Sera from dogs (n=114), including suspect (n=30) and positive (n=50) dogs from a leishmaniasis-endemic area and dogs from a nonendemic area, (n=34), negative for leishmaniasis, were assessed. The results showed that sera from the suspect (42%) and positive (68%) groups responded differently to the antigen titers tested above the cut-off (Optical Density=0.166). This finding suggests that the immune response detected against cyspep may be related to clinical disorders present in these animals. Collectively, the data gathered here suggest that cyspep can sensitize the immune systems of dogs from a leishmaniasis-endemic area to elicit a humoral response, an immunological parameter indicating the contribution of this protein in host-parasite interaction.
The protozoa of the Leishmania genus are the causative agents of leishmaniases, a group of infectious diseases with broad worldwide distribution that are recognized as important public health problems.1 These parasites are transmitted to the mammalian hosts through the bite of female phlebotomine insects during a blood meal, causing cutaneous leishmaniasis (CL) or visceral leishmaniasis (VL) depending on the Leishmania spp. involved. These infections were initially described as zoonotic diseases affecting species of wild, synanthropic and domestic animals caused primarily by Leishmania (Viannia) braziliensis and Leishmania (Leishmania) infantum (syn. L. L. chagasi) in the Americas, which cause CL and VL, respectively; however, humans can also be affected.2,3
There are numerous records of infection in domestic animals (canids, felids, and equids) and among these animals, dogs have been implied as the main reservoirs of LV in the urban transmission cycle in the Americas; however, evidence of their roles as tegumentary leishmaniasis reservoirs is insufficient.4 The clinical outcome of dogs with leishmaniasis is highly variable, since infected dogs present a wide range of signs, varying from being apparently healthy to being severely ill,5 depending on the balance between cellular and humoral immune responses.6
Studies have described aspartic proteinases, metallo proteinases, serine proteinases, and cysteine proteinases as virulence factors in Leishmania spp. acting in many steps of parasitic infection, such as tissue invasion, macrophage survival, and modulation of host immune response.7 Among the proteinases that influence parasite-host interaction, cysteine proteinases have been highlighted and may be powerful targets for studying the immune responses associated with infection by Leishmania spp. In this context, some studies have addressed the cysteine proteinase in humoral and cellular immune responses in canine VL and CL.8–12
Cysteine proteinases (CPs) have been demonstrated to concentrate mainly in amastigote megasomes13 and on the surfaces of promastigotes.14 The major known groups of CPs of Leishmania spp. are the Cathepsin B-like CPs (CPA and CPB enzymes) and the Cathepsin L-like CPs (CPC enzymes), which have the following specific characteristics: (i) CPA enzymes are characterized by a single copy of the gene and by the absence of a long extension of the COOH-terminus prior to its final processing15; (ii) CPB enzymes are characterized by multiple gene copies arranged in tandem sequences whose gene products can vary in their substrate specificity and catalytic properties,16 and they feature a long extension of the COOH-terminus prior to the final processing; and (iii) CPC enzymes are characterized by a single copy of the gene, similar to CPA enzymes, and do not have a COOH-terminal extension.17
The sequence of the COOH-terminal extension of CPB (cyspep) corresponds to approximately 100 amino acids that are released from mature CPB enzymes during processing18,19 into the extracellular environment15 and can be found in the host cell cytoplasm.20 Recent evidence shows that amastigotes and promastigotes of L. (L.) amazonensis release cyspep into the extracellular environment, but only promastigotes release this polypeptide as Pro-CPB.21 Cyspep has been described as highly immunogenic and may influence the immune responses of mice infected with L. (L.) amazonensis,20,22Leishmania (L.) infantum,12 and Leishmania (L.) donovani.23 Furthermore, the activity of T-lymphocyte epitopes from cyspep has been demonstrated in patients with tegumentary leishmaniasis24 and in mice infected with L. (L.) amazonensis.20
Therefore, the aim of this study was to evaluate the reactivity of a recombinant protein based on cyspep (rcyspep) by enzyme-linked immunosorbent assay (ELISA) with sera from dogs of a leishmaniasis-endemic area. Rcyspep reactivity was assessed in sera pooled from positive, suspect, and negative dogs according to prior serological results (DPP® and ELISA-EIE® tests).
Materials and methodsSequence analysisThe amino acid sequence of the COOH-terminal extension of CPB from L. (L.) amazonensis, named cyspep (Nt-APRPVMVEQVIVFDKNCTQGCKKTLIKANECHKNGGGGASMIKCSPQTVTMCTYSNEFCLGGGL LETPDGKCAPYFLGSVINTCHYT-Ct),22 was analyzed using the Basic Local Alignment Search Tool (BLAST) (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and compared with other deposited CPB sequences of Leishmania spp. in the database.
The cyspep sequences were also analyzed for linear (BCPREDS server – http://ailab.ist.psu.edu/bcpred/index.html) and conformational (DiskTope 2.0 server – http://www.cbs.dtu.dk/services/DiscoTope/ and IEDB analyze resource – http://tools.immuneepitope.org/ellipro) epitopes.
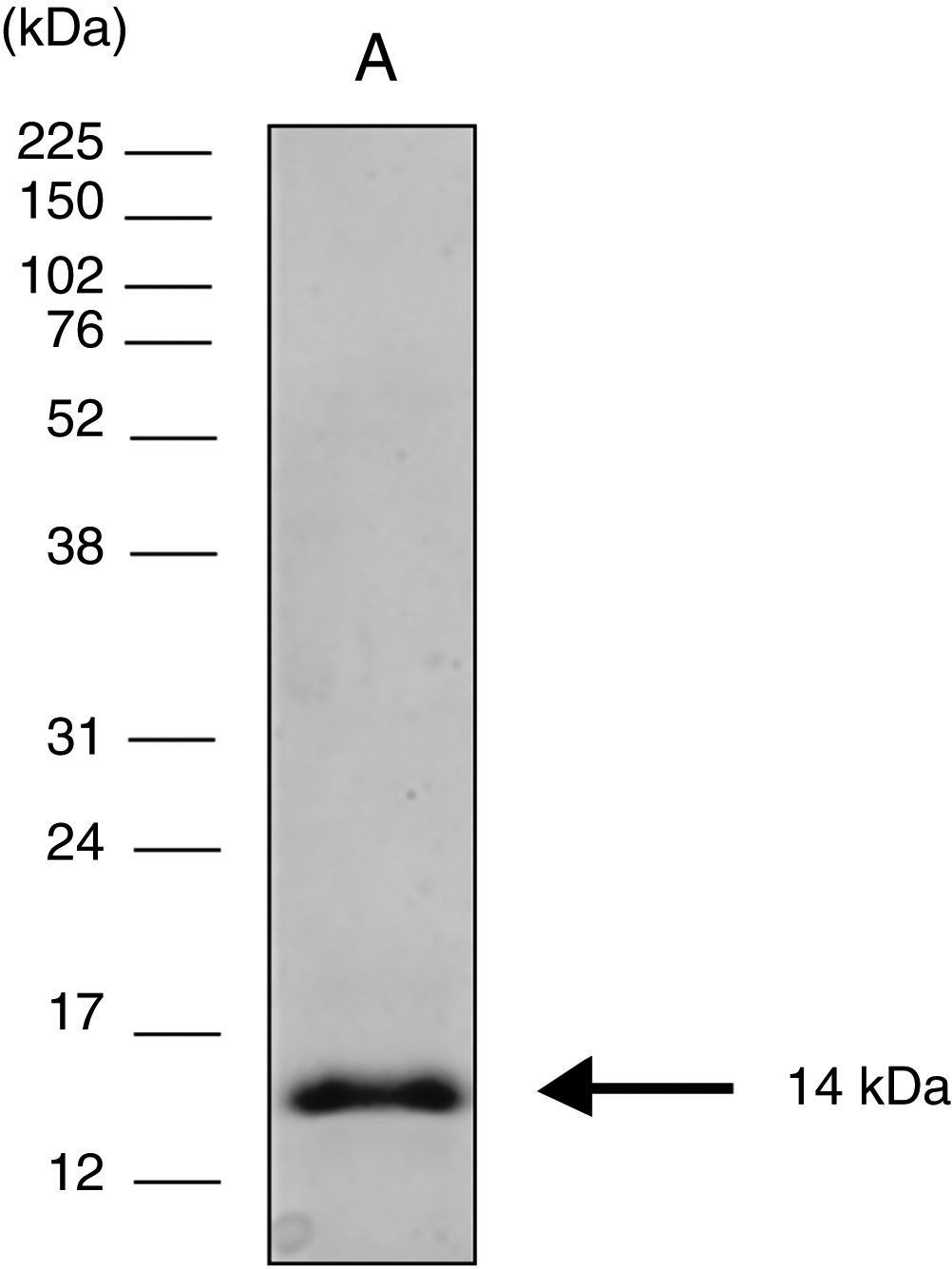
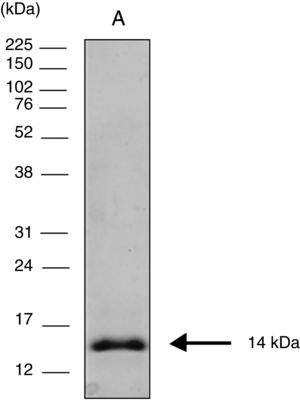
Cloning expression and purification of the COOH-terminal region of CPB rcyspepThe 264-bp fragment corresponding to rcyspep was cloned in the pUC57 vector at the Eco RV endonuclease site and subcloned into the pET28-a vector (+) between the BamHI and Notl sites, and expression and purification of rcyspep were performed according to the QIAexpressionist manual (QIAGEN, Valencia, Santa Clarita, CA, USA), as previously described.21 The purified protein of 14 kDa was stored at −20°C until use.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)Protein samples were analyzed by SDS-PAGE after boiling (3min) with sample buffer [80mM Tris–HCl, pH 6.8, 2% SDS (w/v), 12% glycerol (v/v), 5% β-mercaptoethanol (v/v) and 0.05% bromophenol blue (w/v)]. After electrophoresis (150V at 15mA), the protein bands were visualized using the silver impregnation method with ProteoSilver™ (Sigma–Aldrich Chemical Co., St. Louis, MO, USA). Full-Range Rainbow™ Markers of 12kDa–225kDa (GE Healthcare Life Sciences, Piscataway, NJ, USA) were used to monitor the progress of electrophoresis and to calculate the molecular weights of the assayed protein samples.
Determination of protein concentrationThe protein concentrations of rcyspep were determined using a Micro BCA™ Protein Assay Kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Bovine serum albumin (BSA) was used as a standard.
Serum samples from dogsIn Brazil, serological investigations are performed in dogs of endemic areas, as Visceral Leishmaniasis Surveillance and Control Manual, Ministry of Health.26,27 The collection of samples took place at the municipal health service of the city of Montes Claros. In this work, 114 serum samples from dogs were assayed. Positive and suspect sera from dogs were provided by the Montes Claros Zoonoses Center after screening by DPP™ test and confirmation by ELISA-EIE™ test (Bio-Manguinhos™, Rio de Janeiro, Brazil). Negative control group sera from healthy dogs that presented nonreactive serological tests for Leishmania spp. were provided by the Laboratory of Clinical Analyses-LABORLIFE, Rio de Janeiro. The three study groups were as follows: Group 1 (NEGATIVE) included 34 serum samples from dogs of an nonendemic area for canine leishmaniases that were negative by ELISA-EIE™; Group 2 (SUSPECT) included 30 serum samples from dogs of an area endemic for canine leishmaniases that were positive by the DPP™ serological screening method but negative by ELISA-EIE™; and Group 3 (POSITIVE) included 50 serum samples from dogs positive for canine leishmaniases confirmed by both tests (DPP™ and ELISA-EIE™).
Rcyspep immunoenzymatic assay (ELISA-rcyspep)The cutoff was established from analyses of three standard negative and three positive serum samples on three different plaques against a certain antigen line. The cutoff for each plate was set as the mean of the negative serum readings plus three standard deviations. The tests were performed on 96-well polystyrene plates (Sigma–Aldrich Chemical Co., St. Louis, MO, USA) by sensitization (16h, 4°C) with 100μL (0.5μg/well) of the rcyspep protein in carbonate-bicarbonate buffer (0.05M, pH 9.6). Subsequently, the plate wells were washed (3 times) with wash buffer (0.01M phosphate buffer, pH 7.4, 0.15M sodium chloride, and 0.05% Tween; PBS-T) and incubated (2h, 25°C) with 200μL of blocking buffer (PBS-T containing 5% skim milk). The plates were washed again with PBS-T and incubated (1h, 25°C) with 100μL of dog serum (1:100). After a further wash cycle in PBS-T, the wells were incubated (1h, 25°C) with a peroxidase-conjugated anti-dog IgG antibody (whole molecule, 1:300) produced in rabbit (Sigma–Aldrich Chemical Co., St. Louis, MO, USA). Then, antigen recognition was detected with a citrate-phosphate buffer (0.2M citrate and 0.1M phosphate, pH 5.1) containing 0.4mg/mL OPD and 0.03% hydrogen peroxide for 15min. The reaction was stopped by addition of 50μL of H2SO4, and the optical density was measured on an ELISA reader at 492nm.
Statistical analysisStatistical analyses (cut-off and standard deviation determination) were performed using MedCalc® statistical software.
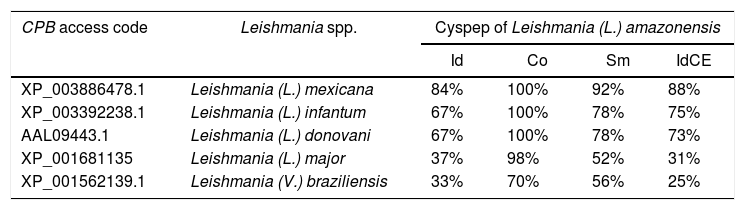
ResultsElectrophoretic cyspep protein profile and in silico analysis of the sequence featuresIn this study, heterologous expression yielded a cyspep protein with a molecular mass of 14kDa, which was confirmed by denaturing electrophoresis and comparison to standard proteins used as markers (Fig. 1). The predicted cyspep protein sequence was further analyzed in silico with the BLAST. This analysis showed that the cyspep sequence has a similarity level with the cyspep proteins of Leishmania spp. between 52% and 92% (Table 1). When comparing the cyspep of L. (L.) amazonensis with L. (L.) infantum and L. (V.) braziliensis, the similarity reached 78% (Table 1).
Comparison of cyspep sequences from Leishmania spp.
| CPB access code | Leishmania spp. | Cyspep of Leishmania (L.) amazonensis | |||
|---|---|---|---|---|---|
| Id | Co | Sm | IdCE | ||
| XP_003886478.1 | Leishmania (L.) mexicana | 84% | 100% | 92% | 88% |
| XP_003392238.1 | Leishmania (L.) infantum | 67% | 100% | 78% | 75% |
| AAL09443.1 | Leishmania (L.) donovani | 67% | 100% | 78% | 73% |
| XP_001681135 | Leishmania (L.) major | 37% | 98% | 52% | 31% |
| XP_001562139.1 | Leishmania (V.) braziliensis | 33% | 70% | 56% | 25% |
Id, identity; Co, coverage; Sm, similarity; IdCE, identity of conformational epitopes.
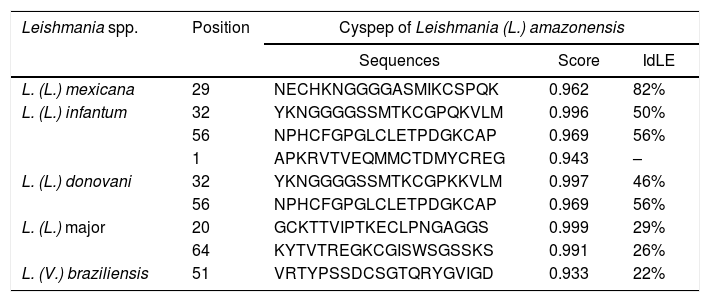
The predicted epitopes of cyspep protein were assessed by in silico assays. This analysis showed that the cyspep sequence had an identity of linear (IdLE) and conformational (IdCE) epitopes level, with the cyspep proteins of Leishmania spp. assayed: IdLE – 22%–82% (Table 2); IdCE – 25%–88%. In these analyses, cyspep of L. (L.) amazonensis shared acceptable levels of identity with L. (L.) infantum (IdLE=50%–56%; IdCE=75%), and with L. (V.) braziliensis (IdLE=22%; IdCE=25%) (Tables 1 and 2). Additionally, the results of these analyses also revealed cyspep sequence of Leishmania spp. had amino acid residues involved in conformational epitopes structuring (Fig. 2).
Prediction of linear epitopes of cyspep of Leishmania spp.
| Leishmania spp. | Position | Cyspep of Leishmania (L.) amazonensis | ||
|---|---|---|---|---|
| Sequences | Score | IdLE | ||
| L. (L.) mexicana | 29 | NECHKNGGGGASMIKCSPQK | 0.962 | 82% |
| L. (L.) infantum | 32 | YKNGGGGSSMTKCGPQKVLM | 0.996 | 50% |
| 56 | NPHCFGPGLCLETPDGKCAP | 0.969 | 56% | |
| 1 | APKRVTVEQMMCTDMYCREG | 0.943 | – | |
| L. (L.) donovani | 32 | YKNGGGGSSMTKCGPKKVLM | 0.997 | 46% |
| 56 | NPHCFGPGLCLETPDGKCAP | 0.969 | 56% | |
| L. (L.) major | 20 | GCKTTVIPTKECLPNGAGGS | 0.999 | 29% |
| 64 | KYTVTREGKCGISWSGSSKS | 0.991 | 26% | |
| L. (V.) braziliensis | 51 | VRTYPSSDCSGTQRYGVIGD | 0.933 | 22% |
IdLE, identity of linear epitopes.
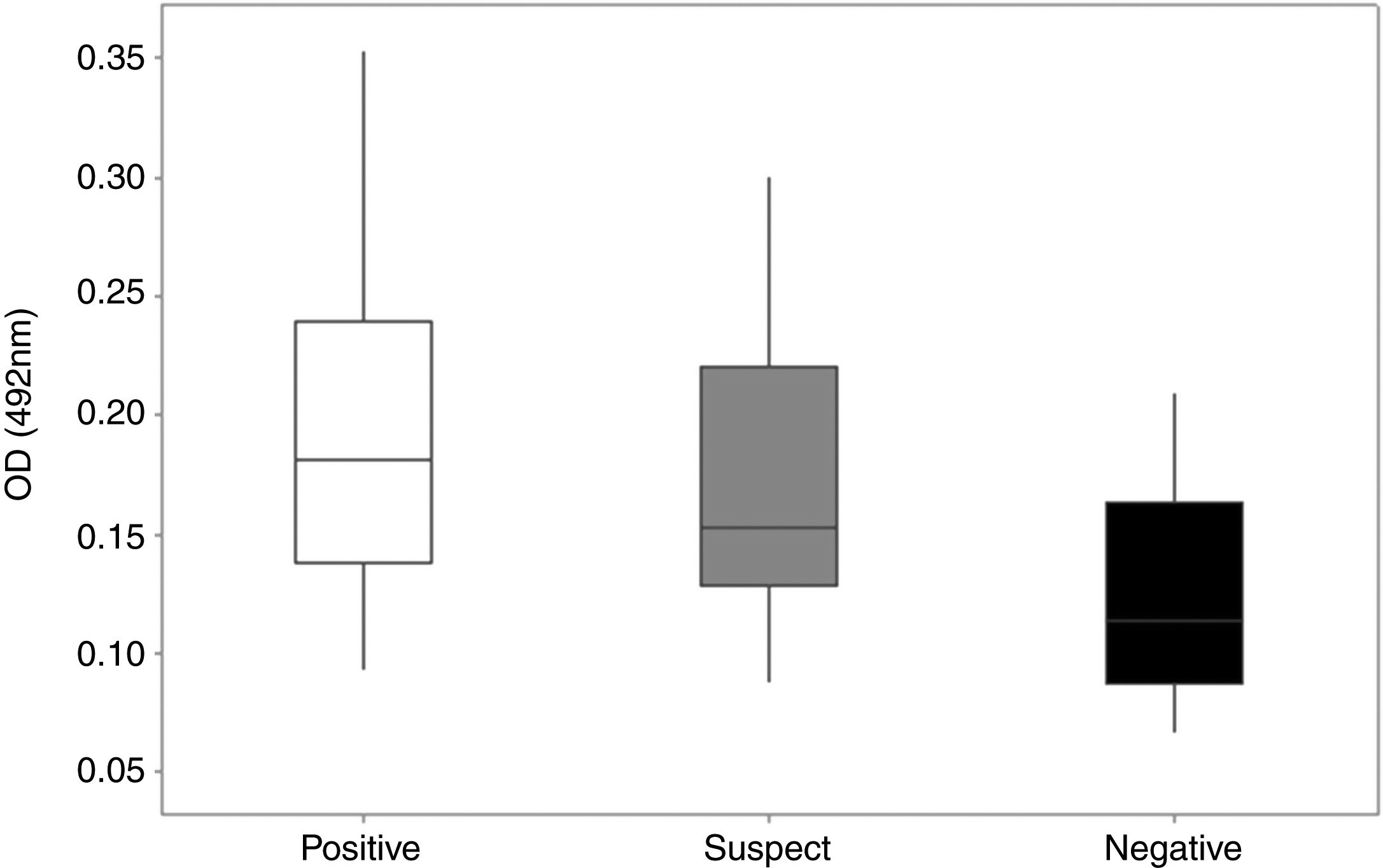
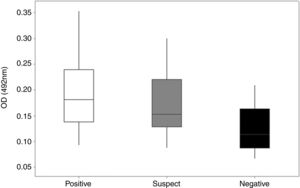
Detection of specific anti-rcyspep IgG antibodies by enzyme-linked immunosorbent assay. The antibody titers were determined in sera from dogs of endemic and nonendemic areas for canine leishmaniasis. The sera were classified as positive (reactive in both DPP and ELISA), suspect (reactive in DPP and negative in ELISA) or negative (negative in both tests). The rcyspep protein was adsorbed to the bottoms of 96-well plates (0.5μg/well)) and allowed to interact with sera. The reactivity of the sera was determined using secondary peroxidase-conjugated rabbit anti-dog IgG antibodies and H2O2/ortho-phenylenediamine by colorimetric reaction, and the well absorbance was measured at 492nm. The results correspond to the mean and standard deviation from three independent assays.
Most cases in Montes Claros, MG, region are cases of L. (L.) infantum and L. (V.) braziliensis infection.27 Rcyspep was used as an antigen for the immunoenzymatic assay to detect specific antibodies in the sera of naturally infected dogs. The results showed that the negative samples from the nonendemic area presented optical density (OD) values between 0.066 and 0.188, indicating a predominance of animals not responding to the test antigen and generating a cut-off point of 0.166.
On the other hand, the suspect and positive groups from endemic areas, presented heterogeneous results in the distribution of serum titers, showing both reactivity and nonreactivity (Fig. 3). The titers of sera from the suspect animal group varied from 0.087 to 0.301 (OD), and approximately 42% (n=14) presented titers above the cut-off value (OD=0.166), suggesting a cyspep antigen-directed immune response in these animals. In the positive group, the titers varied from 0.093 to 0.354 (OD), with 68% (n=34) responsive to the tested antigen.
DiscussionInformation on canine leishmaniases is still scarce, leading to difficulty in understanding the progression and full extent of the infection. Studies on the virulence factors of Leishmania spp. could provide insights, since these factors are directly related to the establishment and outcome of the infection. In mammals, notably humans and laboratory animals, the virulence factors of the parasite have been well described and have been employed to study the infection as well as to promote the development of strategies for leishmaniases control, including recognition of targets for diagnosis, chemotherapy, or vaccines.27
It is well known that the distinct clinical manifestations of leishmaniasis are dependent upon the parasite species and the status of the host's immune system28 and result from interactions between host immune factors and parasite components. Some parasite virulence factors strongly contribute to the pathogenesis profile and enable the parasite to invade and establish infection in mammalian hosts.29,30 This knowledge was the motivation for the present study, which revealed, for the first time, the existence of a canine humoral immune response directed against a recombinant product of one of the best-described virulence factors in leishmaniasis, the CPB protein.7
The combined use of an engineered recombinant antigen and an immunoenzymatic assay is advantageous for the detection and further characterization of specific antibodies against Leishmania infection produced during natural infection in dogs. Such a procedure may be used for qualitative and quantitative specific detection of antibody responses towards virulence factors of this parasite, as assessed in this work for the COOH-terminal region of cysteine protease B of Leishmania spp. In this study, an immunoenzymatic assay established to determine dog antibodies levels specific for cyspep protein confirmed the profiles of the sera tested (negative, positive, and suspect) and indicated additional differences among these serological groups.
Serum samples from dogs of an endemic area in Montes Claros, MG, were considered suitable for the antigenicity evaluation in the immunoenzymatic assay. Despite being considered an endemic region, the municipality of Montes Claros also lacks data on canine infection. Montes Claros presents a favorable environment for the occurrence of leishmaniasis cases since Phlebotominae has been detected in peripheral regions where housing conditions are mostly poor, lacking garbage collection and sanitation, which provides suitable conditions for the transmission of the disease.28 Montes Claros is an endemic area for LV, and between 2010 and 2015, 336 human cases of this disease were reported. Of these, 76 occurred in 2015, and four cases culminated in death. In addition, cutaneous leishmaniasis also occurs in the city, with 437 cases registered in the city during the period from 2010 to 2015.28
This set of findings can be understood based on the complexity of the epidemiological profile of canine infection in an endemic area and the breed-specific antigenicity response variations found in these animals. In Montes Claros, there are at least two Leishmania species transmitted,31,32 and the disease can be the result of a heterogeneous infective inoculum, probably due to multiple independent infections and to differences in dog breeds, which may cause a complex serological response profile in the dog population. Therefore, it is possible that some dogs have a more exacerbated response to cyspep antigen than others, which may explain our findings of serum samples that were both cyspep positive and negative in serological screening or our findings of cyspep positive animals in the suspect group. Furthermore, in epidemiological surveys of canine leishmaniasis, the serological assays commonly used provide insufficient sensitivity and specificity; however, these tests are unable to prove the existence of both parasites and infection.32
As some serum samples (42%; n=14) from the leishmaniasis-suspect dog group were positive for anti-rcyspep IgG, it is reasonable to propose that these dogs produce a humoral immune response against cyspep released as a product of the Leishmania CPB processing. In addition, it is important to comment that additional amino acid residues present in fusion protein did not influence antigenicity results since all serum samples from dogs of a nonendemic area for canine leishmaniases were negative for anti-rcyspep IgG.
Furthermore, the sequences comparisons assays performed here reinforced the antigenicity potential of conserved regions in cyspep protein, for both linear and conformational B lymphocyte epitopes. Thus, the set of cyspep epitopes from Leishmania spp. can be related to the IgG antibodies response detected in dog sera samples. As most cases in the Montes Claros region are cases of L. (L.) infantum and L. (V.) braziliensis infection, it is possible that the assessed dog sera may content antibodies that recognized the linear sequence of amino acids or specific adjacent amino acid strings at different points in folded cyspep protein.25,33,34
Additionally, as cyspep is released by amastigotes during pro-CPB maturation,21 it is plausible that the parasite may use cyspep to establish infection in the dog host by modulating the humoral and cellular immune responses, as proposed for natural human infection20 and experimental mouse infection.22,34 Thus, the clinical presentation and evolution of canine leishmaniasis could be a consequence of interactions between parasite products, such as cyspep, and dog immune components.
ConclusionThis work shows new evidence of the potential use of rcyspep to differentiate infected and uninfected dogs in areas endemic for leishmaniasis. We have found that cyspep can play a role in humoral immune responses in dogs naturally infected with Leishmania spp. Interestingly, some serum samples from infected dogs showed reactivity levels similar to those of samples from the negative group. These data may mean that not all dogs infected in this endemic area produce antibodies against cyspep proteins, possibly due to the clinical features of the animals and/or to variations in CPB expression throughout the disease in dogs. The possibility of rcyspep use as a tool in serological monitoring for canine leishmaniasis is reinforced by recent evidence on a recombinant CPB from L. (V.) braziliensis, and its domains, as serodiagnosis tool of dogs from endemic areas of cutaneous and visceral leishmaniasis in Argentina.8 Further studies still need to be conducted to investigate the potential usefulness of cyspep as a tool in serological monitoring for canine leishmaniasis as well as in discrimination between symptomatic and oligosymptomatic dogs.
FundingThis study was financed in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brasil (CNPq: 301744/2019-0) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. Laura Barral-Veloso is postgraduate fellows at Fiocruz. Carlos R. Alves is a research fellow at CNPq.
Conflicts of interestThe authors declare no conflicts of interest.
We are grateful for the technical and logistic support at the Instituto Oswaldo Cruz Culture Medium Platform and Montes Claros Zoonoses Center.