The reported incidence and fatality rates of SARS-CoV-2 infection in patients receiving maintenance dialysis are higher than those of the general population.
ObjectiveThis study sought to characterize the clinical characteristics and outcomes following COVID-19 infection in this population in a single center in Brazil.
MethodsOut of 497 dialysis patients evaluated between March 1st, 2020 and February 1st, 2021, those presenting symptoms or history of close contact with COVID-19 patients were tested. Disease severity was categorized as mild, moderate, or severe.
ResultsOut of the 497 patients, 8.8% tested positive for COVID-19. These patients were predominantly male (59%), mean age 57.5 ± 17. Hospitalization was required for 45.4% of patients and 15.9% received mechanical ventilation. Symptoms such as fever, cough, dyspnea and asthenia were more frequent in the severe group. Neutrophil to lymphocyte ratio, C- reactive protein, glutamic oxalacetic transaminase and lactic dehydrogenase were significantly higher in the severe group, while hemoglobin and lymphocyte counts were significantly lower. Chest CT >50% of ground glass lesions was the risk factor associated with severe disease and need for hospitalization. The incidence of a thromboembolic event was of 22.7% in this population. The incidence, mortality, and case fatality rates were 954.4/10,000 patients, 151.8/10,000 patients, and 15.9%, respectively.
ConclusionsThe incidence, mortality and case fatality rates in our cohort were significantly higher than those reported for the general population. To institute appropriate control measures and early vaccination in dialysis facilities is imperative to prevent the spread of COVID-19 infection.
The novel coronavirus disease 2019 (COVID-19) is a newly discovered communicable disease caused by the SARS-CoV-2 virus and the most severe clinical COVID-19 scenario is characterized by an inflammatory cytokine storm, resulting in hematological changes and coagulation dysfunction, which can lead to tissue damage and death.1,2
Brazil is among the three countries with the highest number of confirmed cases and deaths due to COVID-19, also one of the lowest per-capita testing rates worldwide. The first COVID-19 case in Brazil was detected on February 26th, 2020, in the city of São Paulo. Until April 2021, almost 14 million confirmed COVID-19 cases with over 378,000 deaths were reported in Brazil, with more than 2.7 million cases and 89,000 deaths in São Paulo state.3
Elderly individuals and persons with underlying chronic illnesses are at a higher risk of death due to COVID-19. Therefore, maintenance dialysis patients who in the vast majority present diabetes mellitus, hypertension, and/or other cardiovascular disease are at a high risk regarding poor outcomes and should be prioritized for COVID-19 vaccination.2
Globally, Brazil is ranked third with respect to the number of chronic dialysis patients (estimated at 133,464), with approximately 92% of in-center hemodialysis (ICHD). Therefore, the establishment of proactive strategies aimed at early diagnosis and appropriate isolation measures to contain COVID-19 infection is imperative until this population is fully vaccined.1,4
The dialysis facility evaluated herein is a regional reference center for the treatment of end stage renal disease (ESRD) and caters to a catchment area of more than 100 cities. ICHD patients require frequent access to the dialysis facility using public transportion and they frequently have close contact with the dialysis staff and other patients which impedes social distancing. This multiple exposure environment increases the risk of COVID-19 infection among ICHD patients, despite preventive measures to minimize the spread of the virus.1
On the other hand, peritoneal dialysis (PD) is a home-based procedure associated with a lower risk of COVID-19, since it entails limited patient contact with hospital and staff members. During the pandemic period, these patients were typically monitored via telemedicine.5
In the present study, we prospectively analyzed the epidemiological and demographic characteristics, clinical features and outcomes of COVID-19 in vulnerable maintenance dialysis patients, grouped according to disease severity. Furthermore, the incidence, mortality, and case fatality rates are compared to those reported in previous studies.
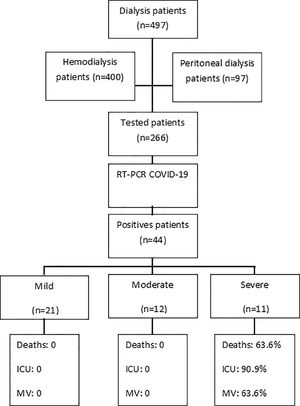
MethodsA total of 497 dialysis patients (400 undergoing HD and 97 undergoing PD) treated at single dialysis facility between March 1st, 2020 and February 1st, 2021 were evaluated. All symptomatic patients or those with a history of close contact with COVID-19 cases at home or during transportation were tested by real-time polymerase chain reaction assay (RT-PCR) using nasopharyngeal swabs. All patients with confirmed RT-PCR results were included in this study (Fig. 1). During the study period, all personnel at the dialysis unit who developed COVID-19 symptoms were also tested by RT-PCR. None of the patients or staff were COVID-19 vaccinated during the study period.
Data for this prospective observational study were obtained from a questionnaire filled out by the patients or their family members and through the online hospital Dialysis Facility Registration System. Written informed consent was obtained and the study protocol was approved by the Medical School (FAMERP) Ethics Committee (#4,212,395).
The COVID-19 positive patients were grouped into three categories based on one of the following clinical characteristics: 1) Mild: patients with mild symptoms, not requiring hospitalization; 2) Moderate: moderate respiratory symptoms, chest computed tomography (CT) presenting 25%–50% ground glass lesions, or patients requiring hospitalization; and 3) Severe: severe symptoms with oxygen saturation <93% or respiratory rate >30 breaths/min, chest CT presenting >50% of ground glass lesions, intensive care unit (ICU) admission, respiratory failure requiring mechanical ventilation (MV), shock, or death.
Statistical analysesQuantitative variables (such as age and duration of treatment) are presented as means ± standard deviation (SD), while categorical variables (such as sex and treatment modality) are presented as frequencies (percentage). Data analyses were performed using the Stats Direct 3.0 software and p < 0.05 values were considered indicative of statistical significance.
ResultsOut of the 497 dialysis patients and 90 staff members, 266 patients and 66 staff members with suspected COVID-19 underwent a nasopharyngeal swab RT-PCR test. Among these, 44/497 (8.8%) patients and 14/90 (15.5%) staff members tested positive for COVID-19, where 40/44 (90.9%) patients were undergoing HD and 4/44 (9%) were undergoing PD.
Patient characteristics are summarized in Table 1. Patients with COVID-19 were predominantly male with a mean age of 57.5 ± 17. Most patients reported contact with COVID-19 confirmed individuals at home (38.6%), although 81.8% of the patients used public transportation to reach the dialysis facility, traveling a mean distance of 41 ± 29 kms. The primary causes of ESRD were hypertensive kidney disease (38.6%) and diabetic nephropathy (29.7%). All patients had at least one of the following coexisting disorders or attributes: hypertension, diabetes mellitus, cardiovascular disease, cancer, chronic obstructive pulmonary disease, and current smokers.
Demographic characteristics of patients with COVID-19 stratified by disease severity.
| Characteristics | Total (n = 44) | Mild (n = 21) | Moderate (n = 12) | Severe (n = 11) | P-value |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 57 ± 17 | 55 ± 20 | 56 ± 12 | 65 ± 16 | 0.27 |
| Age 70 + [n (%)] | 11 (25) | 4 (19) | 2 (16.6) | 5 (45.4) | 0.21 |
| Age 60–69 [n (%)] | 12 (27.2) | 6 (28.5) | 3 (25) | 3 (27.2) | 0.57 |
| Age 41–59 [n (%)] | 13 (29.5) | 5 (23.8) | 6 (50) | 2 (18.1) | 0.12 |
| Age < 40 [n (%)] | 8 (18.1) | 6 (28.5) | 1 (8.3) | 1 (9) | 0.17 |
| Sex, M [n (%)] | 26 (59) | 11 (52.3) | 8 (66.6) | 7 (63.6) | 0.33 |
| Race (W/NW) | 35/9 | 15/6 | 11/1 | 09/02 | 0.18 |
| BMI (kg/m2), mean (SD) | 23.4 ± 4.7 | 22 ± 3.7 | 25.7 ± 5.8 | 24 ± 5.0 | 0.08 |
| Smoker, [n (%)] | 7 (15.9) | 4 (19) | 0 | 3 (27.2) | 0.09 |
| Uses public transportation, [n (%)] | 36 (81.8) | 18 (85.7) | 9 (75) | 7 (63.6) | 0.16 |
| Positive contact, [n (%)] | |||||
| Public transportation | 7 (15.9) | 2 (9.5) | 4 (33.3) | 1 (09) | 0.10 |
| Family members at home | 17 (38.6) | 7 (33.3) | 6 (50) | 4 (36.3) | 0.28 |
| During hospitalization | 4 (9) | 1 (4.7) | 0 | 3 (27.2) | 0.09 |
| Not known | 16 (36.3) | 11 (52.3) | 2 (16.6) | 3 (27.2) | 0.06 |
| Primary causes of ESRD, n (%) | |||||
| Diabetic nephropathy | 13 (29.5) | 5 (23.8) | 6 (50) | 2 (18.1) | 0.14 |
| Hypertensive kidney disease | 17 (38.6) | 6 (28.5) | 4 (33.3) | 7 (63.6) | 0.06 |
| Others | 14 (31.8) | 10 (47.6) | 2 (16.6) | 2 (18.1) | 0.07 |
| Coexisting disorder, n (%) | |||||
| Cardiovascular disease | 19 (43.1) | 9 (42.8) | 6 (50) | 4 (36.3) | 0.51 |
| Hypertension | 39 (88.6) | 19 (90.4) | 11 (91.6) | 9 (81.8) | 0.42 |
| Diabetes mellitus | 21 (47.7) | 7 (33.3) | 07 (58.3) | 7 (63.6) | 0.10 |
| Lung disease | 7 (15.9) | 4 (19) | 1 (8.3) | 2 (18.1) | 0.38 |
| Cancer | 6 (13.6) | 1 (4.7)a | 1 (8.3) | 4 (36.3)a | ap = 0.03 |
| Dialysis modality, n (%) | |||||
| HD | 40 (90) | 21 (100)a | 11 (91.6) | 8 (72.7)a | ap = 0.03 |
| PD | 4 (9) | 0a | 1 (8.3) | 3 (27.2)a | ap = 0.03 |
| Dialysis (months), Mean (SD) | 40.7 ± 45.9 | 35.9 ± 31,8 | 37.2 ± 41.2 | 53.7 ± 71.2 | 0.39 |
| Treatments, n (%) | |||||
| Glucocorticoids | 14 (31.8) | 0a,b | 5 (41.6)a | 9 (81.8)b | ap = 0.003bp = 0.0001 |
| Azithromycin | 32 (72.7) | 13 (61.9) | 9 (75) | 10 (90.9) | 0.11 |
| ACE/ARB | 21 (47.7) | 9 (42.8) | 7 (58.3) | 5 (45.4) | 0.48 |
| Prophylactic Heparin use during hospitalization | 17 (38.6) | 0a,b | 9 (75)a | 8 (72.7)b | ap = 0.0001bp = 0.0001 |
| Oxygen therapy | 16 (36.3) | 0a,b | 5 (41.6)a,c | 11 (100)b,c | ap = 0.003bp = 0.0001cp = 0.004 |
| MV | 7 (15.9) | 0a | 0 | 7 (63.6)a | ap < 0.0001 |
| Hospitalization, n (%) | 20 (45.4) | 0a,b | 9 (75)a,c | 11 (100)b,c | ap < 0.0001bp < 0.0001cp = 0.001 |
| ICU, n (%) | 10 (22.7) | 0 | 0 | 10 (90.9) | ap < 0.0001bp < 0.0001 |
| Thromboembolic event, [n (%)] | 10 (22.7) | 5 (23.8) | 3 (25) | 2 (18.1) | 1.0 |
| *Incidence rate/10,000 | 954.4 | 455.5 | 260.3 | 238.6 | – |
| *Mortality rate/10,000 | 151.8 | 0 | 0 | 151.8 | – |
| *Fatality rate,% | 15.9 | 0 | 0 | 63.6 | – |
M, male; W, white; NW, nonwhite; BMI, body mass index; ESRD, end stage renal disease; HD, hemodialysis; PD, peritoneal dialysis, ACE/ARB, renin angiotensin-aldosterone system (RAAS) inhibitors; MV, Mechanical ventilation; ICU, intensive care unit.
Calculations: The incidence, mortality and case fatality rates were calculated as follows: Incidence = number of cases from 03/01/2020 until the end of the current report (02/01/2021)/number of exposed people per 10,000. Mortality = number of deaths due to COVID-19/number of exposed people per 10,000. Fatality = (number of confirmed deaths due to COVID-19/number of confirmed COVID-19 cases) *100.
Baseline characteristics of patients with mild, moderate, and severe disease were similar; the exception was cancer as a coexisting disorder, which was significantly more frequent in the severe group compared to the mild group (p = 0.03) (Table 1). HD patients presented more frequent mild disease, in contrast to most PD patients, who presented more frequent severe disease (p = 0.03) (Table 1).
The clinical characteristics, laboratory findings and chest CT scan images features at the time of diagnosis are presented in Table 2. The most commonly reported initial symptoms were fever (68.1%), followed by cough (54.5%) and dyspnea (52.2%). Fever (p = 0.04), cough (p = 0.04), dyspnea (p = 0.002) and asthenia (p = 0.004) were significantly more common in the severe group. This severe group also presented significantly higher neutrophil:lymphocyte ratio (N:L), C-reactive protein (CRP), lactic dehydrogenase (LDH), and glutamic oxalacetic transaminase (GOT) and significantly lower hemoglobin and lymphocyte count (Table 2).
Clinical, laboratory and chest CT findings in patients with COVID-19 stratified by disease severity.
CT; computed tomography.
Forty-five percent of the patients who developed COVID-19 required hospitalization (mean hospital stay: 12.8 ± 9 days), 22.7% required intensive care unit (ICU) admission, and 15.9% required mechanical ventilation (MV) (Table 1).
Forty-three percent of the patients developed COVID-19 complications, including acute respiratory distress syndrome (ARDS, 11%), septic shock (20%), bloodstream infection (13%) and cardiovascular complications (7%), while 4% patients required oxygen supplementation post-hospitalization.
Ten patients (22.7) presented a thromboembolic event during the study, comprising six arteriovenous fistula thromboses, one acute limb ischemia- arterial thromboembolism, two pulmonary thrombi embolisms and one kidney transplant venous thrombosis. Comparing patients with and without thromboembolism (TE) event, only the platelet count was higher in the TE group although within normal range (249,800 ± 148,019 vs. 151,617.6 ± 46,984; p = 0.001).
Patients in the moderate and severe groups were significantly more frequently treated with prophylactic heparin during hospitalization than the mild group, with no thromboembolic event difference between the three groups (Table 1). Patients in the severe group were significantly more frequently treated with corticosteroids (81.8%) and required oxygen supplementation (100%) when compared to the other two groups. Previous use of renin-angiotensin-aldosterone system (RAAS) inhibitors (ACE/ARB) was not significantly different between the three groups (Table 1).
Seven deaths were observed among patients with severe COVID-19. The incidence of COVID-19 and the associated mortality and fatality rates in dialysis patients were 954.4/10,000 patients, 151.8/10,000 patients, and 15.9%, respectively (Table 3).
Comparison of national and global COVID-19 incidence, mortality, and fatality rate data.
| COVID-19 dialysis cohorts | Incidence rate/10,000 | Mortality rate/10,000 | Fatality rate,% |
|---|---|---|---|
| The present study | 954.4 | 151.8 | 15.9 |
| Pio-Abreu et al.14 | 341 | 94 | 27.7 |
| Xiong F et al.8 | 215 | 57.3 | 26.6 |
| Goicoechea M et al.10 | 1276 | 390 | 30 |
| Valeri AM et al.15 | – | – | 31 |
| Couchoud C et al.16 | 303 | – | 21 |
| Jager JK et al.17 | – | – | 20 |
| Hsu CM18 | 551 | 137 | 24.9 |
The staff members who tested positive for COVID-19 were placed in home quarantine regardless of the symptoms for a minimum of 10 days, and none required hospitalization, developed complications or died.
In multivariate models, the risk factor associated with severe disease and need for hospitalization due to COVID-19 disease in maintenance dialysis patients was chest CT > 50% of ground glass lesions, with an HR of 25.3 (95% CI 1.17–548.1; p = 0.03).
DiscussionMaintenance dialysis patients are at high risk for COVID-19, and as medications used to reduce COVID-19 hospitalizations and mortality remain elusive, effective and safe vaccines and the implementation of strict measures to reduce virus transmission is imperative to avoid the collapse of dialysis activity.6
As soon as the first COVID-19 case was reported in Brazil, we actively monitored the dialysis patients and staff at our facility and adopted universal masking policies. The first case of COVID-19 among dialysis patients was detected on May 26th and the number of cases peaked in July 2020, with a slight delay in relation to the spread of the disease in the state of São Paulo and in Brazil.3
Even though 81.8% of the patients used public transportation and traveled a considerable distance to reach the dialysis facility, this was a probable risk of contamination for only 15.9% of patients, and most reported household contamination (38.6%).
The demographic characteristics of COVID-19 cases were similar to those observed in the general population with regard to age and comorbidities.7 Clinical features such as cough and dyspnea were also similar, while fever was less frequently detected in our study when compared to the general population (68% vs. 88.7%).7
Symptoms such as fever, asthenia, cough and dyspnea were more frequent in the severe group, consistent with the results of a previous study performed on dialysis patients.8,9
The severe group displayed significantly elevated LDH, CRP, N:L ratio and GOT and lower hemoglobin and lymphocyte count at presentation. In previous studies, laboratory indices associated with worse COVID-19 prognosis included d-dimer, LDH, CRP, thrombocytopenia, lymphopenia and liver dysfunction.8-11 Another study found that the N:L ratio was the major marker associated with severe forms and predicted short-term COVID-19 outcomes in hemodialysis patients.9
Chest CT was performed from the fifth to the seventh day of symptom onset in all patients with COVID-19. As expected, ground-glass lesions with > 50% involvement of the pulmonary parenchyma were more frequent in the severe group and in our study it was the risk factor associated with the severe disease and need for hospitalization.
This study demonstrates the high incidence of thromboembolism events (22.7%) in maintenance dialysis patients post-COVID-19 infection. Chronic kidney disease is a known risk factor for venous thromboembolism events (VTE), occurring 2–4-fold more frequently than in the general population.12 COVID-19 is a disease with high thrombotic risk and anticoagulation protocols have been studied.13 Our study suggests that ESRD patients should be considered as high risk to thrombosis and candidates for prophylactic heparin use even in non-severe presentations.
The high incidence (954.4/10,000 patients), mortality (151.8/10,000 patients), and case fatality rates (15.9%), in our cohort demonstrate the greater vulnerability of dialysis patients to COVID-19 and an almost 6-fold higher risk of death when compared to the Brazilian general population (286.5/10,000, 8/10,000, and 2.8%, respectively).3
A multicenter Brazilian study of HD patients found a lower incidence (341/10,000) and mortality (94/10,000) and higher fatality rates (27.7%), when compared to the present study (Table 3).14 We believe that the higher incidence rate in this study when compared to the aforementioned multicenter Brazilian study is likely attributable to variations in population density, socioeconomic differences, and the timing of the incidence of the disease in different regions.
In contrast, the case fatality rate observed in our study is the lowest reported to date, even when compared to other dialysis patient cohorts (Table 3).8,10,14-18
In our study, despite the lower incidence of COVID-19 among PD patients compared to HD patients (4% vs. 10%) the case fatality rate was much higher among PD patients (50% vs. 12.5%). We recommend due caution when considering strategies of switching patients from HD to PD in an effort to minimize the risk of COVID-19 in HD patients.
It is noteworthy that the percentage of staff members that tested positive for COVID-19 in our study (15.5%) was comparable to that reported in a previous study (12%).19
The limitations of this study include the observational nature of the data, a relatively small sample size, and lack of testing in all patients and staff regardless of symptoms during the study period, due to financial constraints.
The high mortality and case fatality rates observed among dialysis populations reinforce the need to consider these population as high risk for COVID-19 and to institute appropriate control measures and early vaccination to prevent the spread of this infection in this vulnerable group, considering that SARS-CoV-2 vaccine impacts on asymptomatic carrying, transmission and effectiveness in this population are still unknown.
Authors contributionsFSGP the conception and design of the study; FSGP, ACB acquisition of data; HCC, MAF analysis and interpretation of data; FSGP, HCC drafting the article or revising it critically for important intellectual content; MAF final approval of the version to be submitted.
Acknowledgments: None. No funding to declare.