Helicobacter pylori infection can cause gastritis, gastric ulcers, duodenal ulcers, and gastric cancer. Its treatment involves different medications, but resistance to these treatments is increasing. It is currently considered a public health problem.
Aimsto identify regimens used for H. pylori eradication by age group, year of treatment and geographical region of Colombia.
MethodsA cross-sectional study that identified regimens used H. pylori eradication in outpatient consultations over a 6-year period based on a medication dispensing database of 8.5 million people affiliated to the Colombian Health System. The appropriate regimens were those that included a proton pump inhibitor, associated with two antibiotics recommended by clinical practice guidelines (amoxicillin, clarithromycin, levofloxacin, moxifloxacin, tetracycline, doxycycline, metronidazole, tinidazole, and furazolidone).
ResultsA total of 12,011 patients with a diagnosis of acid-peptic disease and H. pylori infection were identified, who had undergone 12,426 eradication treatment courses. Of these, 98.0% used a proton pump inhibitor (PPI), and 91.1% used amoxicillin. A total of 56.1% of the regimens were considered adequate; of these, 42.0% were a combination of PPI, amoxicillin/clarithromycin. This regimen predominated between 2015 and 2017 for all age groups.
ConclusionsThe management of H. pylori infection in the majority of patients is heterogeneous and inconsistent with current recommendations based on evidence of antimicrobial resistance.
Helicobacter pylori (H. pylori) is a Gram-negative flagellate bacterium with spiral shape and microaerophilic behavior.1,2H. pylori infection in Latin America and the Caribbean has overall prevalence reported to be 57%, and 69% in adults.3 In Colombia, the prevalence of H. pylori infection has been documented to be between 77% and 83.0%,4 and infection is currently considered a global public health problem.1
Although many people infected with H. pylori are asymptomatic, the bacterium is responsible for the development of chronic gastritis, functional dyspepsia, gastric or duodenal ulcers, gastric adenocarcinoma and lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma), and it is currently classified as a class I carcinogen.1,5,6 It has also been linked to extra-digestive diseases, including immune thrombocytopenic purpura, unexplained iron deficiency anemia and vitamin B12 deficiency.5,6H. pylori eradication therapy has been shown to be beneficial for the improvement of gastritis and the cure of peptic ulcers and MALT lymphoma and can even prevent the development of gastric cancer.7,8
Treatment of H. pylori infection is based on the use of two or more empirical antibiotics and an acid suppressor, generally a proton pump inhibitor (PPI), which is necessary to maintain a less-acidic pH that facilitates bacterial replication and thus increases the efficacy of antibiotics.9 Among the antibiotics that have been used are amoxicillin, clarithromycin, levofloxacin, nitroimidazoles, tetracycline, doxycycline, furazolidone, and rifabutin.6,9,10 However, the effectiveness of empirical therapy has been decreasing due to increased resistance, mainly to levofloxacin, clarithromycin and metronidazole.5,6,11 As a consequence, treatment regimens with four medications have increased, as have therapies based on bismuth salts.12
In each country or region, it is necessary to investigate treatment regimens and choose the most effective ones13; therefore, local surveillance is necessary to properly select the best eradication regimen according to sensitivity and resistance profiles.11 The Colombian Health System offers universal coverage to the entire population through two regimens, one contributory or paid by workers and employers and another subsidized by the state; these regimes have a benefit plan that includes all drugs used for H. pylori eradication treatment except rifabutin. The objective of this study was to identify the regimens used for H. pylori eradication, by age group, year of treatment and geographical region of Colombia.
Materials and methodsAn observational cross-sectional study of the treatment regimens used in the eradication of H. pylori infection was carried out using data from a drug dispensing database that collects information from approximately 8.5 million people affiliated to the Colombian Health System in six health insurance companies, corresponding to approximately 30.0% of the active affiliated population of the contributory or paid regime and 6.0% of that of the state-subsidized regime, which comprises 16.3% of the Colombian population.
Patients were identified according to International Classification of Diseases (ICD-10) codes indicating gastritis (K290, K291, K293-K297), peptic ulcer (K270-277, K279), gastric ulcer (K250-257, K259), gastrojejunal ulcer (K280-K287, K289), duodenal ulcer (K260-267, K269) and H. pylori infection (B980) as diagnoses. The prescriptions associated with these diagnoses and received by the patients between January 1, 2015, and November 30, 2020, were analyzed. Patients aged 18 years or older of either sex who were seen in outpatient medical consultation and received an antiulcer drug associated with at least one antibiotic were included. Patients who had a diagnosis related to infectious pathologies and those with incomplete data were excluded.
Using the data on the medication consumption of the affiliated population that were systematically obtained by the dispensing company (Audifarma SA), a database was designed that allowed the following groups of patient variables to be collected:
- 1.
Sociodemographic: Sex, age (groups: 18-29 years, 30-49 years, 50-64 years and 85 years), city of dispensation.
- 2.
Geographic areas: Place of residence was categorized by department according to the regions of Colombia and the classification of the National Administrative Department of Statistics (Departamento Administrativo Nacional de Estadística - DANE) of Colombia, as follows: The Bogotá-Cundinamarca region, Caribbean region, Central region, Eastern region, Pacific region and Amazon-Orinoquía region.
- 3.
Type of prescribing physician: General practitioner, specialist related to the management of acid-peptic disease (gastroenterology, general surgery, internal medicine and family medicine), and other specialists (cardiology, pulmonology, etc.).
- 4.
Antiulcer drugs: PPIs (omeprazole, esomeprazole, lansoprazole, dexlansoprazole, pantoprazole, levo-pantoprazole and rabeprazole), H2 receptor antagonists (ranitidine, famotidine, cimetidine), gastric protectors (sucralfate, bismuth salts), and antacids (aluminum hydroxide, magnesium hydroxide, other).
- 5.
Antibiotics indicated for eradication regimens for H. pylori: Amoxicillin, clarithromycin, levofloxacin, moxifloxacin, tetracycline, doxycycline, metronidazole, tinidazole, furazolidone and rifabutin (not available).
- 6.
Inadequate regimens: Regimens that did not include a proton pump inhibitor, regimens that included a single antibiotic or regimens that included antibiotics not indicated for eradication regimens for H. pylori according to the recommendations of the clinical practice guidelines (azithromycin, erythromycin, ciprofloxacin, trimethoprim-sulfamethoxazole, clavulanic acid and ampicillin).
The protocol was approved by the Bioethics Committee of the Universidad Tecnológica de Pereira (Technological University of Pereira) in the category of risk-free research (Code: CBE22-19). The ethical principles established by the Declaration of Helsinki were respected.
The data were analyzed with the statistical package SPSS Statistics, version 26.0 for Windows (IBM, USA). A descriptive analysis was performed with frequencies and proportions for the qualitative variables and measures of central tendency and dispersion for the quantitative variables, depending on the parametric behavior established by the Kolmogorov-Smirnov test.
ResultsA total of 12,011 patients who received some eradication treatment for H. pylori, distributed throughout 132 different cities, were identified. A total of 65.5% (n = 7863) of the patients were women, and the median age was 49.9 years (interquartile range: 38.4-60.7 years; range: 18.0-100.5 years). A total of 10.7% (n = 1290) of the patients were between 18 and 29 years old, 39.4% (n = 4727) between 30 and 49 years old, 33.4% (4013) between 50 and 64 years old, and 16.5% (n = 1981) were 65 or older. The majority of the patients were in the Bogotá-Cundinamarca region (n = 5722; 47.6%), followed by the Caribbean region (n = 2424; 20.2%), Pacific region (n = 1592; 13.3%), Central region (n = 1461; 12.2%) and Amazon-Orinoquía-Eastern region (n = 812; 6.8%). A total of 95.2% (n = 11,434) of the patients participated in the contributory regime, and 4.8% (n = 577) belonged to the subsidized regime.
Antiulcer drugs and antibioticsA total of 12,426 H. pylori eradication treatment regimens were found. The most frequently prescribed group of antiulcer drugs was PPIs (n = 12175; 98.0%), followed by antacids (n = 1645; 13.2%), sucralfate (n = 526; 4.2%) and H2 receptor antagonists (n = 186; 1.5%). Regarding antibiotics, amoxicillin was the most commonly used (n = 11,314; 91.1%), followed by clarithromycin (n = 3,889; 31.3%) and metronidazole (n = 2,865; 23.1%), tinidazole (n = 1022; 8.2%), doxycycline (n = 464; 3.7%), furazolidone (n = 418; 3.4%), tetracycline (n = 295; 2.4%) and levofloxacin (n = 252; 2.0%). Bismuth salts were prescribed in 0.2% (n = 20) of the regimens.
H pylori treatment regimensMost of the treatment regimens were prescribed by general practitioners (n = 10,594; 85.3%), followed by specialists related to the management of acid-peptic disease (n = 1529; 12.3%) and other clinical or surgical specialists (n = 303; 2.4%).
A total of 43.9% (n = 5453) of the treatment regimens were inadequate - 37.7% (n = 4680) because they included only one antibiotic, 4.8% (n = 596) included antibiotics that were not recommended, and 2.0% (n = 251) did not include PPIs. The most frequent inappropriate regimens were PPI-amoxicillin (n = 4415; 81.0% of inappropriate regimens), PPI-metronidazole-ciprofloxacin (n = 341; 6.3%), PPI-clarithromycin (n = 115; 2.1%), PPI-amoxicillin-erythromycin (n = 80; 1.5%) and PPI-levofloxacin (n = 67; 1.2%).
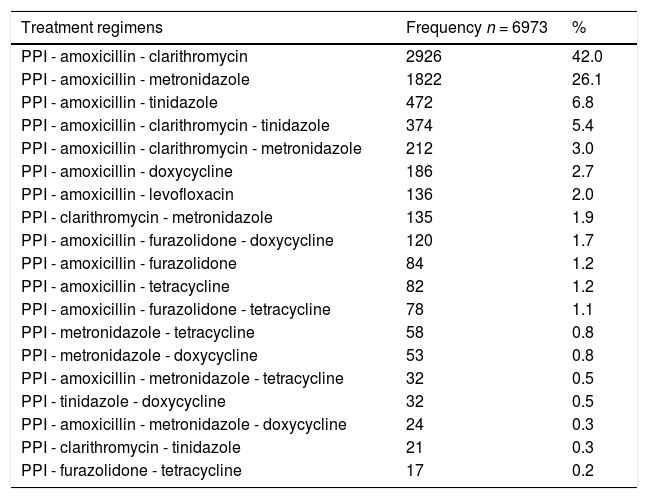
A total of 6973 (56.1%) adequate treatment regimens included a PPI and two or more antibiotics recommended for the eradication of H. pylori; these were distributed in 45 different combinations. The most frequent combination was a PPI-amoxicillin-clarithromycin (n = 2926; 42.0%) (see Table 1).
Main adequate eradication regimens for H. pylori, Colombia 2015-2020.
PPI: Proton-pump inhibitor.
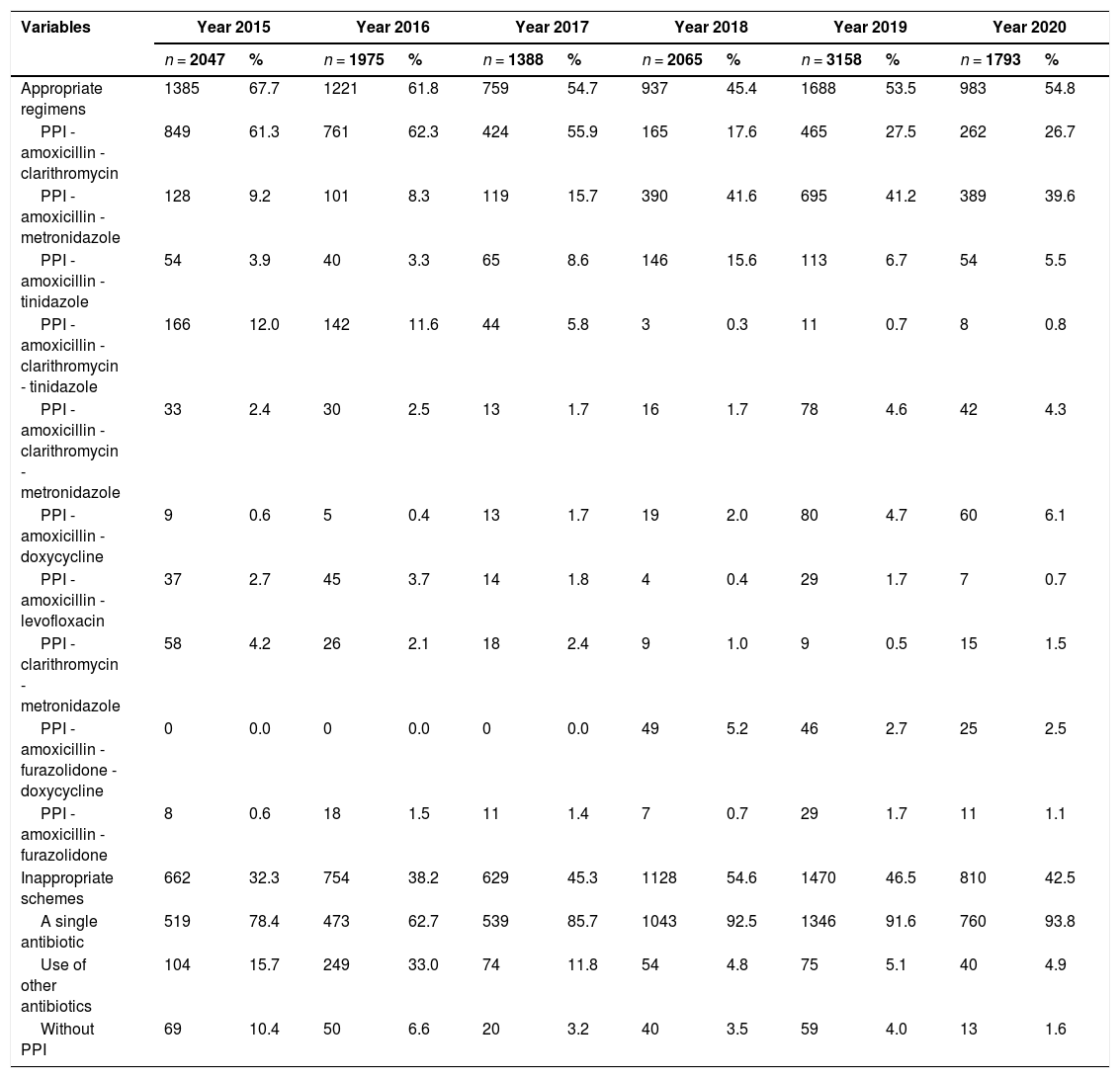
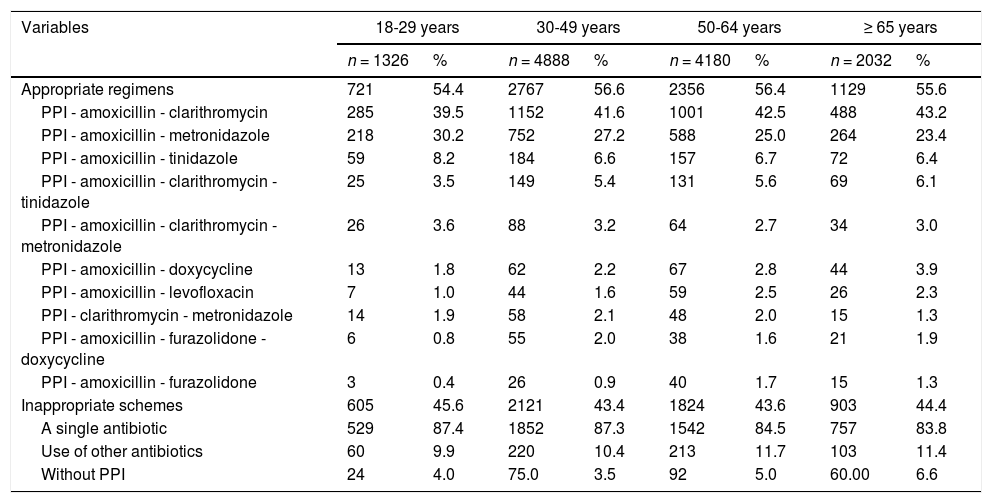
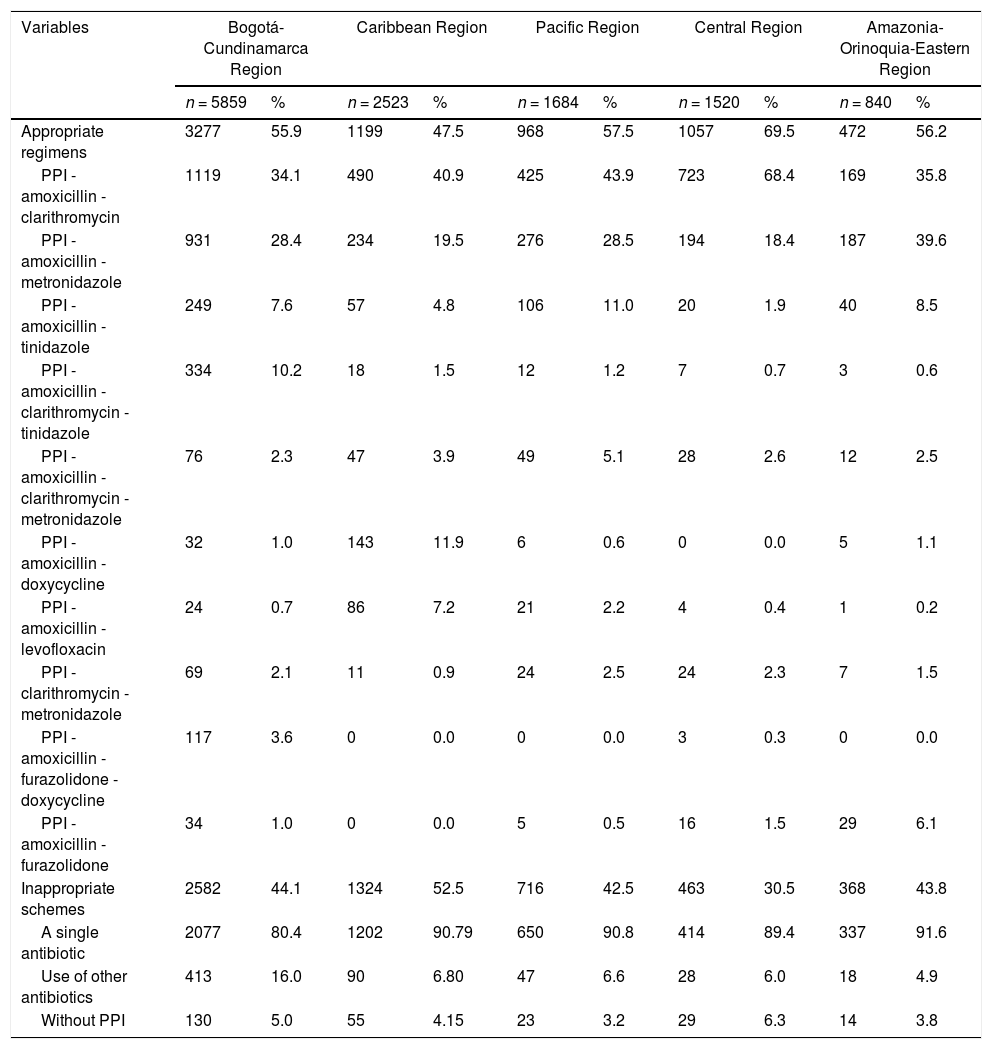
Between 2015 and 2017, the most commonly used treatment regimen was PPI-amoxicillin-clarithromycin, while between 2018 and 2020, the predominant regimen was PPI-amoxicillin-metronidazole (Table 2). For all age groups, the most commonly used regimen was PPI-amoxicillin-clarithromycin (Table 3). In terms of geographic region, the central region had the highest proportion of adequate regimens; in contrast, the Caribbean region had the highest frequency of inadequate regimens. In addition, the use of PPI-amoxicillin-clarithromycin predominated in all regions except the Amazon-Orinoquía-Eastern region, where PPI-amoxicillin-metronidazole prevailed (Table 4).
Characterization of 12,426 H. pylori eradication regimens according to the year of treatment, Colombia 2015-2020.
PPI: Proton-pump inhibitor.
Characterization of 12,426 H. pylori eradication regimens according to age groups, Colombia 2015-2020.
PPI: Proton-pump inhibitor
Characterization of 12,426 H. pylori eradication regimens according to geographic regions, Colombia 2015-2020.
PPI: Proton-pump inhibitor.
This study allowed us to characterize the medications received by patients affiliated to the Colombian Health System who were diagnosed with gastritis, gastric ulcer or duodenal ulcer and had indications for H. pylori eradication treatment according to age group, geographical region of the country and year of treatment (2015 - 2020). These findings can be useful for decision-making among healthcare, academic and scientific personnel regarding treatment needs and risks among patients, especially under conditions in which antibiotic resistance of H. pylori is high and is associated with an increased risk of therapeutic failure.5,8,11
The mean age of the patients was similar to that found in other studies (48.5-53.1 years).4,7,14,15 There was a predominance of women, as documented in different European countries (53.8-59.2%)15,16 and in Colombia (65.0%),4 but contrasted with findings from Asian countries such as China and Korea, where H. pylori infection predominantly affects men (53.9-56.8%).7,17 The prevalence in women in most studies is probably due to iron deficiency, which is more common in women18 and associated with an increased risk of H. pylori infection.19
Regarding the prescription of antibiotics for the treatment of H. pylori, a predominance of amoxicillin and clarithromycin was found, consistent with that reported in other studies.14,16,20 However, metronidazole is rarely used in Sweden (0.01%),16 while in China it is used in almost one-fifth of patients,14 which is slightly more consistent with this report and lower than what was previously described in Colombia.20 Prescription patterns are especially dependent on the availability of the drug in each country, its inclusion in the health care system, and the antimicrobial resistance in each region,17 which can be influenced by the easy access to antibiotics in Latin America, self-medication, lax regulation of sales21 and the fact that these drugs are widely used in daily medical consultations22 not only for gastrointestinal diseases but also for respiratory, gynecological and urinary infections, among others.14
In general, the most commonly used treatment regimen in most regions of the country was triple therapy with a PPI combined with amoxicillin and clarithromycin (42.0%), consistent with the findings of a study conducted in 17 European countries (39.4%)15 and reports for the USA (27-53%)23 and, at lower proportions, for Sweden (21.4%).16 In China, in contrast, the most widely used regimen was PPI combined with clarithromycin and metronidazole (24.6%). In a study conducted in Colombia, researchers found that patients were treated mainly with a PPI combined with amoxicillin and levofloxacin (41.9%).4 This triple therapy is recommended by management guidelines when resistance to clarithromycin is lower than 15%.6,9,10 However, it may not be the best option in all geographic regions of the country, especially where the average resistance reported is 18% (95% CI: 7-31%)21 and when there are few studies on sensitivity and resistance of H. pylori in the different regions of Colombia; additionally, this information is unknown for many areas of the country,24 but there are reports showing an effectiveness of only 72%.25 Therefore, clinicians may be using standard triple therapy in areas where the regimen is ineffective, as occurs in several European countries.26
It is even more worrying that since 2018, the association of PPIs with amoxicillin and metronidazole began to predominate in Colombia, in contrast with to the low frequency of use in other countries (2.6-3.1%).14,15 The use of metronidazole is very striking, especially when resistance to this drug in the country has been identified as 83% (95% CI: 73-90%),21 much higher than the average found in Latin America (53%)21 and in Europe and Asia (38-61%).11 Clearly, it should not be considered an optimal treatment regimen in the country. According to the Maastricht V consensus, quadruple therapy with bismuth salts, PPIs and a combination of two antibiotics - amoxicillin, tetracycline, furazolidone or metronidazole - has shown cure rates greater than 90% for of H. pylori strains resistant to clarithromycin, metronidazole and fluoroquinolones6 and should be used based on resistance patterns and eradication rates present in each geographic region.21,24 The Colombian guide even recommends the use of quadruple bismuth therapy in these cases.9 On the other hand, there is evidence in the country that eradication regimens containing doxycycline can have good efficacy; thus, for example, hybrid therapy made up of PPI and amoxicillin for 15 days, and bismuth salts and doxycycline in the last 10 days, presented an eradication rate of 94.6% in patients treated for the first time and of 90.2% in patients with ineffective prior treatment.27 However, more studies are required in the country,9 even more so when in Spain it was evidenced that quadruple therapy containing doxycycline was less effective than quadruple therapy with tetracycline,28 despite being rarely used in the country.29
Although local and international clinical practice guidelines recommend combining bismuth salts with different management regimens to improve eradication rates,6,9,10 this analysis found that they were prescribed for only 0.2% of patients. This is in marked contrast with reports for different European countries, where bismuth salts were prescribed for 9.4% of patients,15 and is not consistent with a previous Colombian study, in which 648 general practitioners and specialists were interviewed, and 16.5% of them indicated that they used bismuth salts in the eradication regimens they prescribed.20 In Colombia, bismuth subsalicylate has a current health record according to the National Institute of Medicines and Food Surveillance (Instituto Nacional de Vigilancia de Medicamentos y Alimentos - INVIMA).30 Therefore, its use should be promoted by physicians who manage patients with H. pylori infection, especially when there is evidence of H. pylori resistance to first-line antibiotics.21,24
On the other hand, 43.9% of treatment regimens were considered unsuitable as they included a PPI combined with a single antibiotic (amoxicillin, clarithromycin, levofloxacin, among others) or with antibiotics that were not indicated in the management guidelines or because they included other antiulcer drugs. This situation has been reported in other countries14,31 and was an issue in Colombia some years ago.20 In China, Li et al. found that 25.6% of patients with H. pylori infection received treatment regimens that were not indicated.14 In Spain, Laredo et al. reported that only 34.2% of the regimens prescribed by general practitioners were appropriate, compared to 73.6% of those prescribed by gastroenterologists (p < 0.001).31 Finally, in Colombia, Gómez et al. interviewed general practitioners, internists and gastroenterologists in nine cities and found that 20.0% used a single antibiotic in treatment regimens and that 2.6% did not use PPIs.20 Although antimicrobial resistance is the main factor responsible for treatment success, decisions regarding the prescription of antibiotics also play an important role,14 as does adherence to clinical practice guidelines.6,9,10 In addition to the above, it is possible that the proportion of inadequate regimens in the country is higher, due to factors such as the use of low doses of PPIs, inadequate doses of antibiotics or treatment regimens lasting less than 10 days,6,9,10 conditions that could not be identified in this report. Furthermore, the fact of using antibiotics recommended by clinical practice guidelines is not necessarily a proxy of using a correct regimen, since this will also depend on the resistance and sensitivity patterns of H. pylori in each region, a factor that would increase the proportion of inadequate regimens, especially when they have eradication rates below 90%.6,9,10
However, some limitations in the interpretation of the results are recognized. Access to the medical records was not obtained to verify the methods used to diagnose the patients, the requested paraclinical results or the confirmation of eradication. Similarly, it is unknown whether the patients took medications that were prescribed outside the health care system or were not delivered by the dispensing company. In addition, the duration of treatment and the dosage interval in the management regimens were not considered. However, the study included a significant number of patients distributed throughout most of the national territory and included participants in both the contributory and subsidized regimes.
ConclusionsWith these findings, we can conclude that the management of H. pylori infection in the majority of patients is heterogeneous and inconsistent with current recommendations. More than one-fifth of patients received treatment regimens involving metronidazole, which carries a high risk of therapeutic failure. Bismuth salts were used by a minimum number of patients despite being one of the drugs recommended to improve effectiveness of eradication regimens. More studies are required in the country to address this important public health problem, especially research that shows the eradication rates associated with the different management regimens.
Declaration of funding sourcesThe present study did not receive funding.
Ethics approval and consent to participateThe protocol was approved by the Bioethics Committee of the Universidad Tecnológica de Pereira (Technological University of Pereira) in the category of risk-free research (Code: CBE22-19). The ethical principles established by the Declaration of Helsinki were respected.
Availability of data and materialprotocols.io
Data repository: dx.doi.org/10.17504/protocols.io.btdvni66
CRediT authorship contribution statementLuis Fernando Valladales-Restrepo: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft. Yessenia Correa-Sánchez: Formal analysis, Investigation, Data curation. Brayan Stiven Aristizábal-Carmona: Formal analysis, Investigation, Data curation. Jorge Enrique Machado-Alba: Methodology, Validation, Formal analysis, Resources, Writing – review & editing, Supervision.
Soffy Claritza López for her work in creating the database.