Inflammatory bowel disease (IBD) is a chronic disorder involving the gastrointestinal tract. Immunosuppressive drugs are usually prescribed to treat IBD patients, and this treatment can lead to tuberculosis reactivation. This paper aimed to analyze tuberculin skin test (TST) results in IBD patients at a reference center in Brazil.
MethodsWe evaluated TST results in IBD patients using a cross-sectional study. We also analyzed the medical records of patients treated at a reference IBD outpatient unit where TST is routinely performed.
ResultsWe reviewed 119 medical records of 57 (47.9%) Crohn's disease (CD), 57 (47.9%) ulcerative colitis (UC) and 5 (4.2%) indeterminate colitis (IC) patients. The mean (SD) age was 43.5 (13.7) years old. TST was positive in 24 (20.2%) of the patients. TST was positive in 16/57 (28.1%) UC and 6/57 (10.5%) CD patients (prevalence ratio [PR] 2.7). Forty-one patients (34.5%) were taking immunosuppressive drugs (azathioprine or prednisone) at the time of the TST, and six of these patients (14.6%) had positive test results. Two patients using infliximab had negative TST results. Thirty-five of the 41 patients (85.4%) on immunosuppressive treatment were anergic compared with 73.1% (57/78) of the untreated patients (PR 1.2).
ConclusionsPatients with IBD have TST results similar to the general Brazilian population. Within the IBD population, CD patients have a lower frequency of TST positivity than UC patients.
Inflammatory bowel disease (IBD) is a chronic disorder with three subtypes, namely Crohn's disease (CD), ulcerative colitis (UC), and indeterminate colitis (IC). All three diseases occur throughout the world and represent a serious health problem. IBD patients have a compromised quality of life and may develop complications.1 To control IBD, medicines such as aminosalicylates, corticosteroids, immunosuppressive agents and biological therapies are prescribed during treatment. These drugs are provided free-of-charge by the Brazilian Health Department.2 In Brazil, the Ministry of Health did not routinely release anti-tumor necrosis factor (anti-TNF) for treating patients with UC at the time of this study.
There are nearly 70,000 new cases of tuberculosis (TB) in Brazil per year. In the state of Bahia more than 4000 annual cases of the disease have been reported. Despite the slow and constant decline in the incidence of TB in recent years, there have been approximately 4600 deaths in the state of Bahia, which is a very high number, considering that TB is relatively simple to diagnose, is curable, and the treatment is provided for free by the public health system.3 Infected patients form granulomas that prevent the bacillus from multiplying and disseminating, leading to the development of latent tuberculosis (LTBI). The reactivation of LTBI may occur with the use of immunosuppressive therapy and presence of diseases that cause immune deficiencies.4,5 The tuberculin skin test (TST) is widely used as a screening tool for LTBI. Treatment is recommended for LTBI in patients who have a TST result equal to or greater than 5mm in addition to having a concurrent risk factor, such as HIV infection, immunosuppression, contact with pulmonary TB within the last two years by patients who had received the bacille Calmette-Guérin (BCG) vaccine more than two years previously, untreated TB with sequelae of lesions visible on a chest X-ray or organ transplantion. Treatment is also recommended for patients with a TST result equal to or greater than 10mm with the following risk factors: contact with pulmonary TB within the last two years by patients who had received the BCG vaccine more than two years previously, intravenous drug use, immunocompromise, or having indigenous ancestry.6
Drugs such as corticosteroids, azathioprine and monoclonal anti-TNF can cause reactivation of LTBI.7,8 The Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU) reported that LTBI must tracked not only in patients taking anti-TNF-α but rather in all patients on immunosuppressive medication.9 Prednisone reduces activation and proliferation of lymphocytes, inhibits the production of certain cytokines, such as IL-1, IL-2, IL-6, IL-11 and TNF α, causes lymphopenia and monocytopenia, and inhibits the differentiation of monocytes into macrophages. Azathioprine can cause leukopenia, inhibit NK and CD8+ cytotoxic T cells and stimulate T cell apoptosis. Both drugs can cause anergy in patients and lead to false-negative TST.10 A one-month steroid treatment at doses greater than 15mg/day may lead to anergic states and false-negative TST results. Similar results have been observed with an azathioprine dosage greater than 2mg/kg/day.10
This study aimed to analyze TST results in IBD patients at a reference center in Brazil.
MethodsStudy designThis study was conducted in the Inflammatory Bowel Disease Unit of Roberto Santos General Hospital in Salvador city. This clinic is a reference center in the state of Bahia for IBD diagnosis and treatment. The study was approved by the Ethics Committee of Bahia School of Medicine and Public Health, protocol number 139/2008. This was a cross-sectional study carried on by analyzing patient records under regular follow-up care. A total of 119 patients from January to March of 2009 were analyzed. We evaluated the following variables: age, gender, length of time experiencing symptoms, and length of time of IBD diagnosis when the TST was performed, IBD Montreal classification, IBD treatment and TST and chest X-ray results.
PatientsPatients with a clinical, radiological, endoscopic and histological diagnosis of IBD on regular follow-up (last visit within the last six months) were included in this study. We see approximately 30 patients per week in this outpatient clinic. Patients were excluded from the study if they had cancer, HIV infection, or other clinical conditions that could alter the TST results. All of the TST values were analyzed and classified as follows: negative (TST less than 5mm), weakly positive (TST equal to or greater than 5mm but less than 10mm), or strongly reactive (TST equal to or greater than 10mm). Patients were classified as positive or negative after reviewing their TST scores and considering the following risk factors: use of immunosuppressive drugs, use of prednisone greater than 15mg/day for at least one month, use of azathioprine greater than 2mg/kg/day, use of anti-TNF-α, chest X-ray consistent with past TB, and diabetes mellitus. Patients had a positive TST if the TST result was ≥10mm alone or ≥5mm with at least one of the risk factors listed above.11 The CD and UC patients were classified according to the Montreal classification.12
Statistical analysisData collected were entered into a database in SPSS version 16.0. Initially, a descriptive analysis was performed on the sample. Given the characteristics of the study design, it was not possible to estimate the appropriate standard error, therefore, no inferential statistics were calculated (p-values and confidence intervals).13 Proportions were used for categorical variables, and means with their standard deviations were used for continuous variables. A comparison between the proportions was performed using the prevalence ratio (PR).
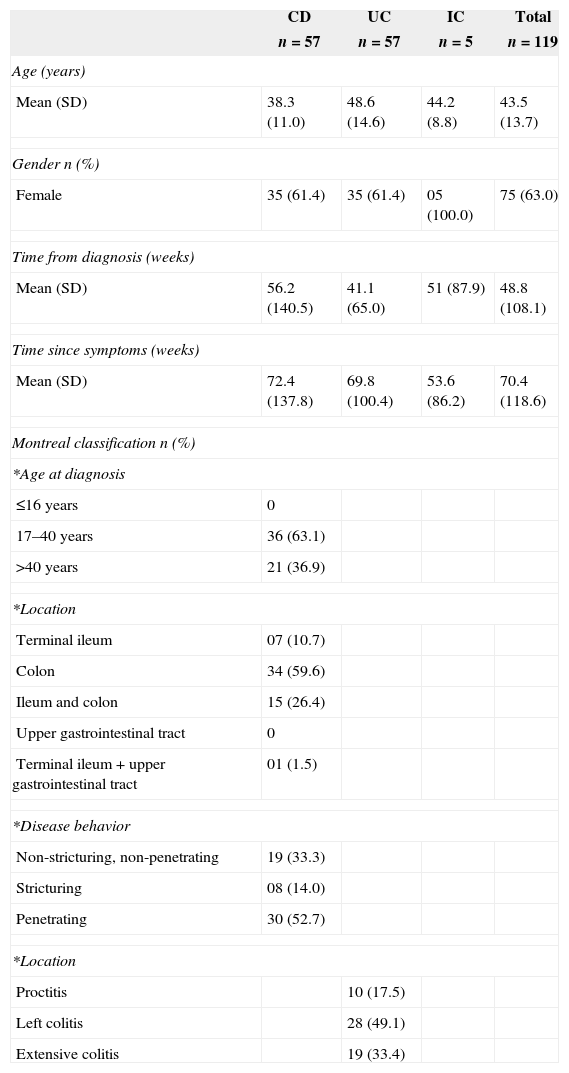
ResultsDemographic and clinical characteristics are summarized in Table 1. The characterization of the TST according to CD and UC diagnosis is shown in Table 2. Three patients with penetrating disease (50%), two patients with stricturing disease (33.3%) and one patient with non-stricturing and non-penetrating disease (16.7%) were TST positive.
Clinical and demographic characteristics of inflammatory bowel disease patients.
| CD | UC | IC | Total | |
|---|---|---|---|---|
| n=57 | n=57 | n=5 | n=119 | |
| Age (years) | ||||
| Mean (SD) | 38.3 (11.0) | 48.6 (14.6) | 44.2 (8.8) | 43.5 (13.7) |
| Gender n (%) | ||||
| Female | 35 (61.4) | 35 (61.4) | 05 (100.0) | 75 (63.0) |
| Time from diagnosis (weeks) | ||||
| Mean (SD) | 56.2 (140.5) | 41.1 (65.0) | 51 (87.9) | 48.8 (108.1) |
| Time since symptoms (weeks) | ||||
| Mean (SD) | 72.4 (137.8) | 69.8 (100.4) | 53.6 (86.2) | 70.4 (118.6) |
| Montreal classification n (%) | ||||
| *Age at diagnosis | ||||
| ≤16 years | 0 | |||
| 17–40 years | 36 (63.1) | |||
| >40 years | 21 (36.9) | |||
| *Location | ||||
| Terminal ileum | 07 (10.7) | |||
| Colon | 34 (59.6) | |||
| Ileum and colon | 15 (26.4) | |||
| Upper gastrointestinal tract | 0 | |||
| Terminal ileum+upper gastrointestinal tract | 01 (1.5) | |||
| *Disease behavior | ||||
| Non-stricturing, non-penetrating | 19 (33.3) | |||
| Stricturing | 08 (14.0) | |||
| Penetrating | 30 (52.7) | |||
| *Location | ||||
| Proctitis | 10 (17.5) | |||
| Left colitis | 28 (49.1) | |||
| Extensive colitis | 19 (33.4) | |||
CD, Crohn's disease; UC, ulcerative colitis; IC, indeterminate colitis.
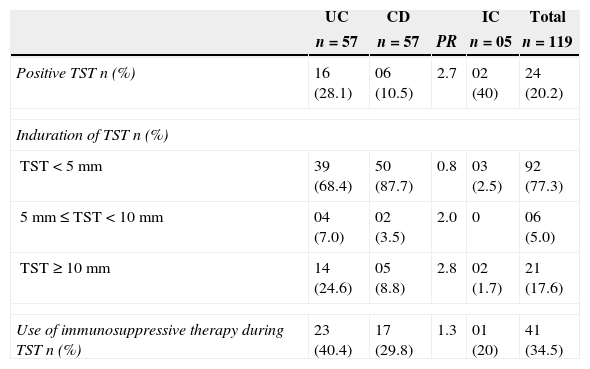
Characterization of TST results and immunosuppressive therapy according to inflammatory bowel disease.
| UC | CD | IC | Total | ||
|---|---|---|---|---|---|
| n=57 | n=57 | PR | n=05 | n=119 | |
| Positive TST n (%) | 16 (28.1) | 06 (10.5) | 2.7 | 02 (40) | 24 (20.2) |
| Induration of TST n (%) | |||||
| TST<5mm | 39 (68.4) | 50 (87.7) | 0.8 | 03 (2.5) | 92 (77.3) |
| 5mm≤TST<10mm | 04 (7.0) | 02 (3.5) | 2.0 | 0 | 06 (5.0) |
| TST≥10mm | 14 (24.6) | 05 (8.8) | 2.8 | 02 (1.7) | 21 (17.6) |
| Use of immunosuppressive therapy during TST n (%) | 23 (40.4) | 17 (29.8) | 1.3 | 01 (20) | 41 (34.5) |
CD, Crohn's disease; UC, ulcerative colitis; IC, indeterminate colitis; PR, prevalence ratio.
Overall, 24 patients (20.2%) were TST positive. The TST induration was less than 5mm in 92 patients (77.3%), equal to or more than 5mm but less than 10mm in six patients (5%) and equal to or greater than 10mm in 21 patients (17.6%). Of the 119 patients, 41 (34.5%) were taking immunosuppressive drugs (azathioprine or prednisone) at the time of the TST, and 14.6% (6/41) of these patients were TST positive. The two patients on infliximab were both TST negative and had normal chest X-rays. No patients taking both drugs (prednisone and azathioprine) presented a TST equal to or greater than 5mm. Thirty-five (85.4%) of the 41 immunosuppressed patients were anergic compared with 57 (73.1%) of the 78 untreated patients (PR 1.2). Seven patients received prophylactic treatment with isoniazid before the use of azathioprine. Of these, six patients were TST positive, and one patient had negative TST, but the chest X-ray was suggestive of TBLI.
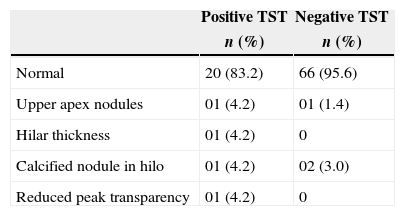
A chest X-ray was performed on 93 patients. The chest X-ray features are summarized in Table 3.
DiscussionThe majority of the patients in this study were female, which is consistent with the literature showing a predominance of female patients in the IBD population.14 Age at diagnosis of CD patients in our study is in line with other studies indicating that most patients were younger than 40 years old.15 This study sample had an increased frequency of penetrating disease. The explanation for this finding is that the study was conducted at a reference center.
In Brazil, the Ministry of Health has reported that 5–20% of the general population is TST positive.16 According to these data, the frequency of positive TST in the general Brazilian population is similar to that encountered in our IBD patients. The use of immunosuppressive therapy is expected to cause some degree of anergy. However, our study did obtain positive TST results, indicating that LTBI is present in this population. In another study in northeast Brazil, the prevalence of positive TST in patients with chronic diseases was 22%.17
Mow et al.18 evaluated TST results from 69 IBD patients and demonstrated that 83% (48/69) of the patients treated with corticosteroids and/or other immunosuppressive therapy (excluding infliximab) were anergic compared with 43% (21/69) of untreated patients (p<0.002). One possible explanation for this finding is that TST is not an effective screening method for patients with LTBI and IBD because IBD patients use immunosuppressive therapies, which can lead to anergy. In our study, no patients using both prednisone and azathioprine were strong reactive to TST. These results reinforce the theory that simultaneous use of both immunosuppressive drugs increases the risk of anergy and interferes with TST, causing false-negative results.19 The frequency of anergy in patients on immunosuppressive therapy found in our study was similar to the frequency reported in previous studies. The high rates of anergy found in patients not using immunosuppressive therapy could be related to their nutritional status or be characteristic of IBD.
CD patients presented a lower frequency of overall positive TST results and had a higher frequency of TST results less than 5mm compared with UC patients. However, the use of immunosuppressive therapy was equivalent between CD and UC patients. This suggests that the immunodeficiency in CD patients could influence the TST results. CD is associated with an imbalance of activated Treg/Th1 and Th17 cell populations. A lack of appropriate T cell regulation or the over-production of effector T cells may be involved in CD development and exacerbation.20 The abnormal function of Treg cells in CD patients could lead to a reduced response to delayed hypersensitivity. Although the TST has some limitations, its utilization as a diagnostic test for TB in IBD patients is still recommended.
Chest X-rays have a sensitivity of 59–82% and specificity of 52–63% for active TB. Furthermore, chest X-rays only demonstrate abnormality in 10–20% of patients with LTBI.21 However, in our study, chest X-rays identified LTBI in three patients who had a negative TST. Our data reinforce the notion that TST alone is insufficient for the detection of LTBI. Therefore, TST in association with a chest X-ray and a detailed history of TB exposure are essential for TB screening in IBD patients.
A study by Gomez-Reino et al.22 resulted in 3573 negative TST results in a population of 4593 rheumatoid arthritis patients receiving infliximab. A repeated TST in 2196 initially negative patients identified 168 positive patients; 8% of the patients initially classified as TST negative were positive on a repeated test. In the Brazilian state of Mato Grosso do Sul, among the 194 participants, 65 patients (33.5%) were strong PPD reactors in the first test, and 129 were weak reactors or non-reactors. They underwent a second test, which revealed that 10 more patients were reactors. Hence, the booster effect rate was 7.8%. Repeating TST may improve the LTBI detection rate and could be a method for optimizing the TST process.23 Some authors have recommended repeating the TST to increase the accuracy of the results (booster effect). Repeated testing is recommended for patients with initial TST results less than 10mm.24 It is possible that if we repeated the TST in our sample, additional positive LTBI patients would have been identified. This possible outcome reinforces the importance of the TST booster concept in immunosuppressed patients.
A new test has recently emerged as an additional opportunity to screen for LTBI infections. The QuantiFERON test measures a component of cell-mediated immunity in response to stimulation by Mycobacterium tuberculosis. This test quantifies the production of interferon-gamma in the blood and was approved by the FDA in 2001. In 2003 and 2005, the QuantiFERON test had its use and recommendations defined by the CDC in the United States.25 A study conducted in 200826 evaluated TST and QuantiFERON efficacy in patients with IBD. In contrast to QuantiFERON, TST was negatively influenced by immunosuppression and previous BCG vaccinations. The authors suggested that TST must be replaced by QuantiFERON as a screening test for LTBI in IBD patients on immunosuppressive therapy. Nevertheless, a meta-analysis showed that it remains difficult to determine superiority between the interferon-gamma release assay and TST because both tests are negatively affected by immunosuppressive therapy. Therefore, screening prior to the initiation of immunosuppressive therapy should be considered, and it is imperative that all patients receive screening prior to initiating anti-TNF therapy.27 Currently, the QuantiFERON test is not available for population screening in Brazil.
ConclusionsIn conclusion, our patients with IBD presented TST results similar to the results of the general Brazilian population. CD patients had a lower frequency of positive TST than UC patients, which reinforced the importance of including additional methods, such as chest X-rays or other exams, when screening for LTBI in this population. Additional studies are required to evaluate the booster effect and QuantiFERON in IBD patients from endemic areas, considering the high risk of disseminated TB during treatment.
Conflicts of interestThe authors declare no conflicts of interest.