Usual treatment regimens with vancomycin often fail to provide adequate serum levels in patients with severe infections.
MethodsRetrospective analysis of vancomycin trough serum measurements. The following parameters were calculated by Bayesian analysis: vancomycin clearance, distribution volume, and peak estimated concentrations. The area under the concentration curve (AUC) (total daily dose/24h clearance of vancomycin) was used to determine the effectiveness of treatment through the ratio of AUC/minimum inhibitory concentration (MIC) above 400, using MIC=1μg/mL, based on isolates of Staphylococci in cultures.
ResultsSixty-one vancomycin trough measurements were analyzed in 31 patients. AUC/MIC>400 was obtained in 34 out of 61 dosages (55.7%), but the mean vancomycin dose required to achieve these levels was 81mg/kg/day. In cases where the usual doses were administered (40–60mg/kg/day), AUC/MIC>400 was obtained in nine out of 18 dosages (50%), in 13 patients. Trough serum concentrations above 15mg/L presented a positive predictive value of 100% and a negative predictive value of 71% for AUC/MIC>400.
ConclusionHigher than usual vancomycin doses may be required to treat staphylococcal infections in children with oncologic/hematologic diseases. Since the best known predictor of efficacy is the AUC/MIC ratio, serum trough concentrations must be analyzed in conjunction with MICs of prevalent Staphylococci and pharmacokinetic tools such as Bayesian analysis.
Children with cancer suffer the effects of immune suppression caused by chemotherapy and by the disease itself. Prolonged hospitalizations, frequent use of broad-spectrum antibiotics, the need for long-term central venous catheters, and loss of mucous membrane and skin integrity also contribute to elevated risk of severe infections.1 Among neutropenic patients, 15% to 25% develop bloodstream infections and, frequently, they also develop infections of the respiratory and urinary tracts, skin, and digestive system.2 Available data show that Gram-positive bacteria cause 45% to 70% of the bloodstream infections in neutropenic patients. The most commonly isolated Gram-positive bacteria are coagulase-negative staphylococci and Staphylococcus aureus, besides Enterococcus.3 Almost all Staphylococcus strains isolated from cancer patients are methicillin-resistant, and vancomycin is the drug of choice for these infections. Teicoplanin, a glycopeptide whose effectiveness is similar to vancomycin, still has a limited use due to higher cost. New and expensive agents with proven efficacy against staphylococci, such as linezolid, quinupristine-dalfopristine, and tigecycline also have limited data regarding use in children.4
Vancomycin exhibits a complex pharmacokinetics, with time-dependent bactericidal effect and moderate post-antibiotic effect. It also has poor tissue penetration, and some authors question its effectiveness in severe infections.5,6 The consensus is that the obtainment of serum free-vancomycin levels four to five times the minimal inhibitory concentration (MIC) or a ratio of area under curve (AUC) of concentration versus time/MIC above 400 is essential to achieve bacterial eradication and clinical success.7 For methicillin-resistant Staphylococcus aureus strains with MIC above 2μg/mL, vancomycin is not effective; considering the binding to serum proteins of around 50%, trough levels of vancomycin should be 15 to 20μg/mL to reliably eradicate strains with a MIC of 2μg/mL. The trough level is the minimal serum concentration obtained within 30minutes to one hour before the subsequent dose, after drug serum stabilization (usually after the fourth dose). Only a fraction (20% to 30%) of the vancomycin serum concentration can be obtained in pulmonary tissue, so higher doses are required to treat pneumonia.4,8 Other sites, such as bones, cardiac vegetations, and tissues around prosthetic devices, also have limited penetration.9
The usual vancomycin regimens frequently fail to provide minimal serum levels of 15μg/mL. Kitzis and Goldstein analyzed serum concentrations in adults (n=1,737) who received usual doses of 2 to 6g/day. Adequate levels to treat susceptible methicillin-resistant Staphylococcus aureus (MIC up to 2μg/mL) were obtained in 81% of the patients, but only in 20.9% to treat intermediate-sensitivity strains (MIC 2–8μg/mL). In 780 patients, levels under 10μg/mL were observed 36 to 48hours after the beginning of treatment.10 Alternative regimens which could optimize the pharmacodynamics have been proposed, such as elevation of the intermittent doses and continuous infusion.11 Administration of higher doses can provide effective serum levels, but the risk of nephrotoxicity may be unacceptable with doses higher than 4g/day in adults.12
The vancomycin ideal therapeutic regimen for cancer patients has not yet been determined and has been poorly studied. The aims of this study were to analyze the vancomycin estimated clearance and obtained serum levels in children and adolescents with cancer, in order to evaluate the suitability of usual dosing regimens for this population.
MethodsThis was a retrospective study, including oncologic/hematologic patients, hospitalized at the intensive care unit of this hospital, or submitted to bone marrow transplantation, who used vancomycin in the year 2010. The study was approved by the institutional research ethics committee (protocol number 1913/2010). Because of its retrospective characteristics, the informed consent was waived.
Vancomycin was measured with a particle enhanced turbidimetric inhibition immunoassay: free vancomycin in the serum competes with particle-bound vancomycin for drug-specific antibody binding sites, thereby inhibiting antibody-mediated particle aggregation. The rate and amount of particle aggregation are inversely proportional to the amount of vancomycin present in the sample.13 Blood samples were obtained one hour before the subsequent dose, in steady-state, which is at least after the fourth dose of vancomycin. Using anthropometric data and vancomycin trough measurements, the following parameters were calculated by Bayesian analysis: vancomycin clearance, distribution volume, and peak estimated concentration. Calculations were made using an open-source pharmacokinetics software (JPKD–College of Pharmacy, Kaoshiung Medical University–Taiwan). The area under the curve (AUC) of vancomycin concentration, defined as total daily dose/24h vancomycin clearance, was analyzed. The effectiveness of the regimen was evaluated by obtaining an AUC/MIC ratio above 400. The value of MIC used for calculations was the mode (i.e., the most frequent value) of all MICs obtained from isolated Staphylococcus strains in culture samples of study patients. MIC was determined by agar diffusion or automated MIC (BD Phoenix™ Automated Microbiology System). The Statistical Package for Social Sciences (SPSS) version 10.1 (Pearson's correlation test and descriptive statistics) and Microsoft Excel (linear regression and graphics) softwares were used for statistical analysis.
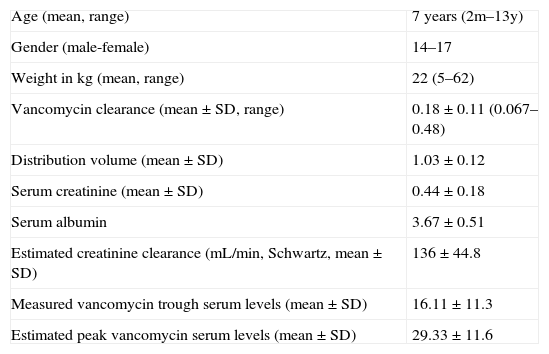
ResultsSixty-one vancomycin serum levels were analyzed in 31 patients. The general characteristics, clearance values, and vancomycin concentrations are depicted in Table 1. Diagnoses of patients are shown in Table 2.
Characteristics of the pediatric oncology/hematology patients.
| Age (mean, range) | 7 years (2m–13y) |
| Gender (male-female) | 14–17 |
| Weight in kg (mean, range) | 22 (5–62) |
| Vancomycin clearance (mean±SD, range) | 0.18±0.11 (0.067–0.48) |
| Distribution volume (mean±SD) | 1.03±0.12 |
| Serum creatinine (mean±SD) | 0.44±0.18 |
| Serum albumin | 3.67±0.51 |
| Estimated creatinine clearance (mL/min, Schwartz, mean±SD) | 136±44.8 |
| Measured vancomycin trough serum levels (mean±SD) | 16.11±11.3 |
| Estimated peak vancomycin serum levels (mean±SD) | 29.33±11.6 |
SD, standard deviation.
Oncology/hematology diagnoses (n).
| Acute lymphoblastic leukemia+bone marrow transplantation (2) |
| Acute lymphoblastic leukemia (6) |
| Acute myeloblastic leukemia (3) |
| Adrenal cortical carcinoma (1) |
| Astrocytoma (1) |
| B-cell lymphoma (1) |
| Botryoid rhabdomyosarcoma (1) |
| Brainstem primitive neuroectodermal tumor (2) |
| Burkitt lymphoma (1) |
| Craniopharyngioma (2) |
| Ependymoma (1) |
| Fanconi anemia+allogeneic BMT (1) |
| Forearm rhabdomyosarcoma (1) |
| Hepatoblastoma (1) |
| Infantile fibrosarcoma (1) |
| Medullary aplasia+allogeneic BMT (1) |
| Medulloblastoma (1) |
| Neuroblastoma+Pepper syndrome (1) |
| Optic nerve glioma (1) |
| Oropharynx undifferentiated carcinoma (1) |
| Rhinopharynx carcinoma (1) |
Coagulase-negative staphylococci were isolated from seven blood cultures and in two skin wound cultures. S. aureus was isolated from two blood cultures and in one tracheal aspirate sample. Other staphylococci isolated from blood cultures were S. epidermidis (2), S. hominis (1), and S. haemolyticus (2). Of all isolates, only four were methicillin-sensitive. The MICs observed ranged from 0.5 to 1.5, and the most frequent value was 1μg/mL, found in seven isolates. This was the mode value used in the analysis of the AUC/MIC quotient.
AUC/MIC results >400 were obtained in 34 of 61 dosages (55.7%), but the mean vancomycin dose to achieve this ratio was 81mg/kg/day (range: 10–156mg/kg/day). When the usual vancomycin dose was administered (40–60mg/kg/day), AUC/MIC>400 was obtained in nine of 18 dosages (50%), from 13 patients.
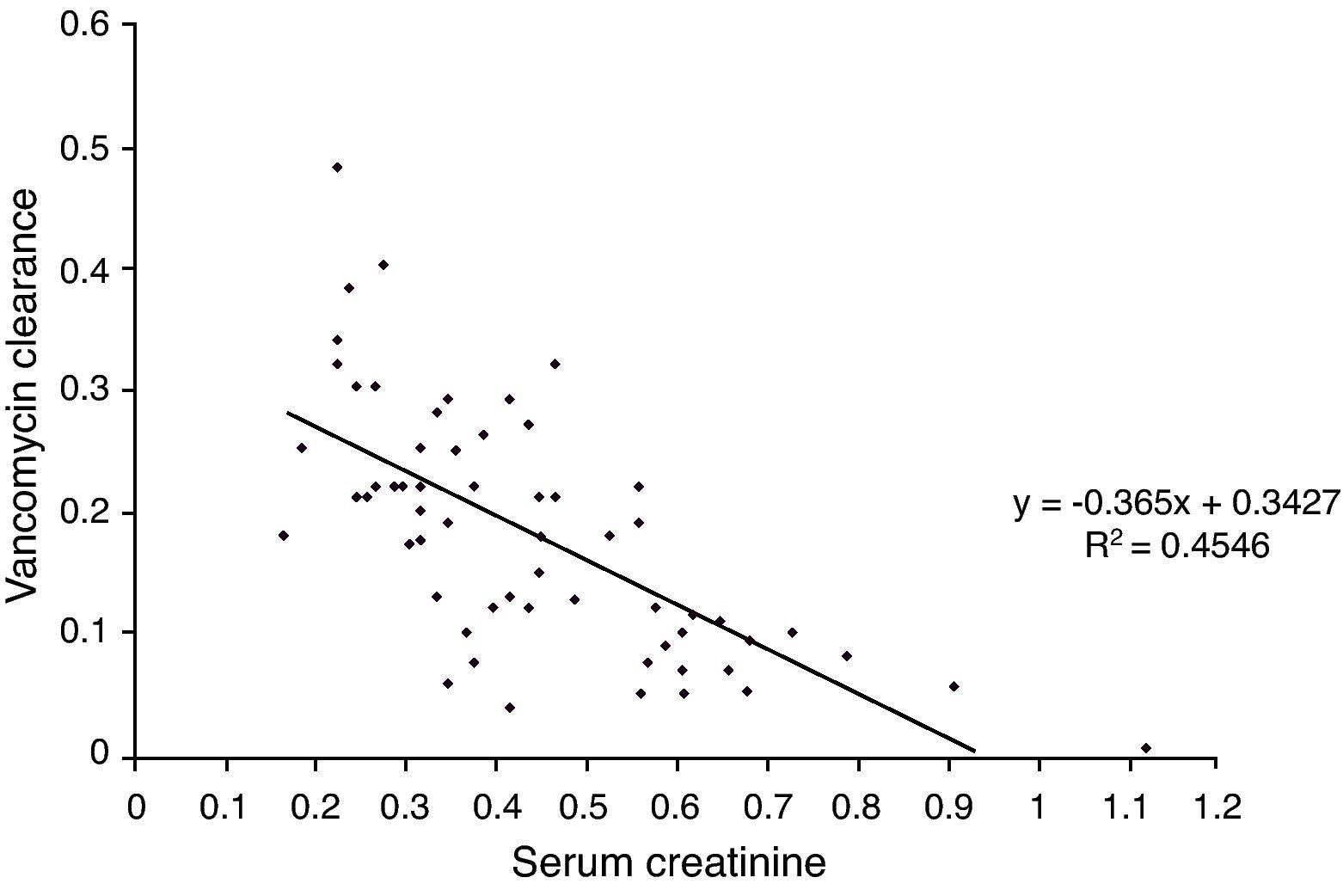
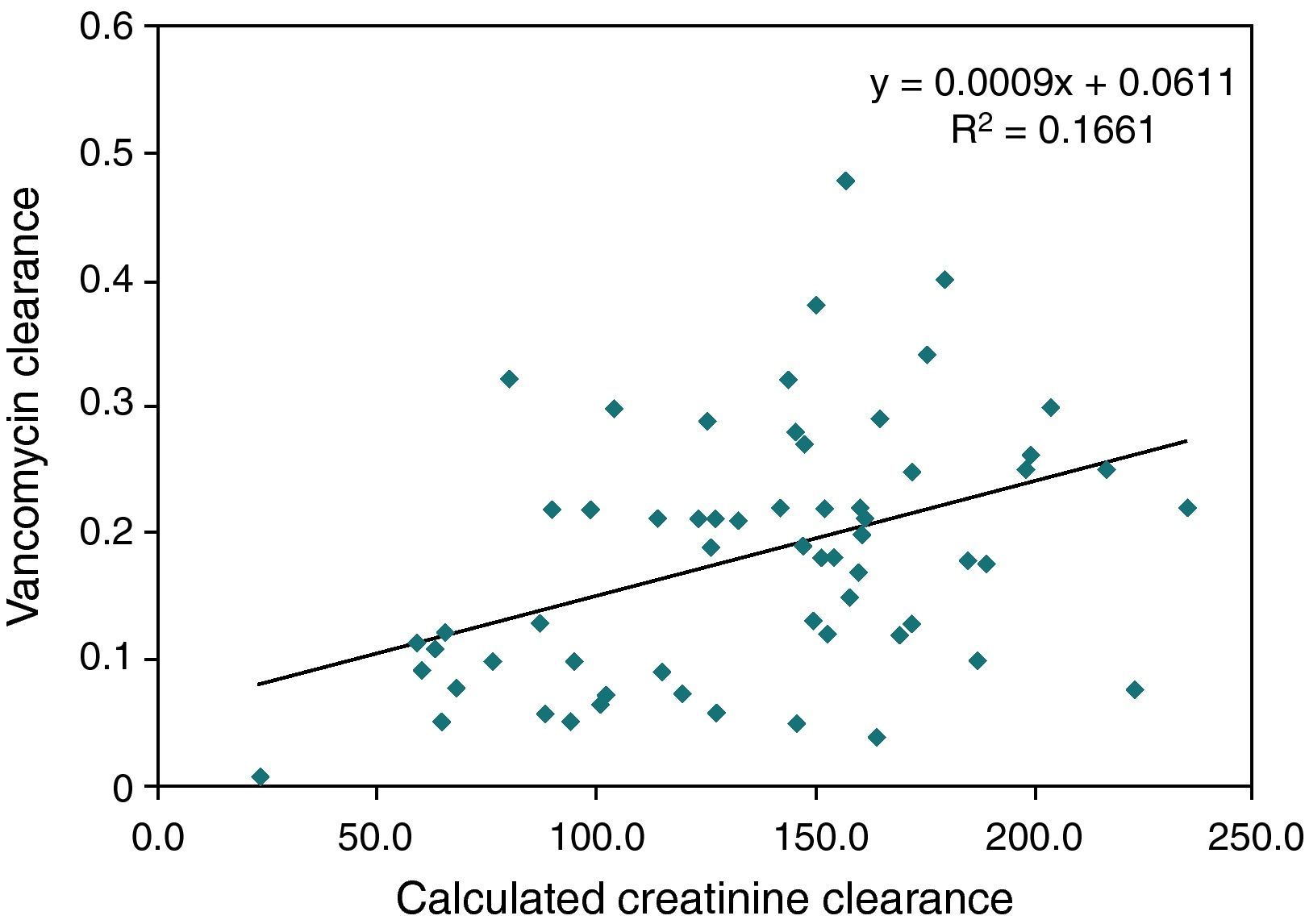
AUC/MIC>400 was observed on 11 occasions in which the trough levels were below 15mg/L (18%). However, in all instances where levels were above 15mg/L, the AUC/MIC was always >400, i.e., trough levels above 15mg/L have a positive predictive value of 100% and a negative predictive value of 71% for the population studied and at current rates of bacterial sensitivity to MIC of 1μg/mL. The calculated vancomycin clearance by Bayesian analysis presented a stronger correlation with serum creatinine levels (Pearson's correlation coefficient: −0.67, p<0.0001) than with creatinine clearance levels estimated with the Schwartz formula14 (Pearson's correlation coefficient: 0.40, p=0.001). Figs. 1 and 2 show the linear adjustment lines (simple regression). The best-fit line equation of vancomycin clearance in relation to creatinine was: vancomycin clearance=(−0.365×serum creatinine)+0.3427. This equation could predict the values of vancomycin clearance using serum creatinine with a ±20% precision in 41% of the analyzed cases. No significant correlation between albumin levels and vancomycin clearance or distribution volume was observed.
Two patients (6.4%) had increased creatinine levels above 50% with vancomycin doses higher than usual. The greatest change was observed in an 8-year-old patient with baseline creatinine of 0.4, which rose to 0.73 with a vancomycin dose of 83mg/kg/d.
DiscussionPiro et al. analyzed vancomycin serum levels in 56 oncologic patients, submitted to bone marrow transplantation, and observed that the number of patients with under-therapeutic levels was significantly higher than those who achieved therapeutic levels. These authors concluded that higher doses are needed in oncologic patients with normal renal function.15 This fact can be explained by what was observed in the present patient sample, which demonstrated vancomycin clearances and distribution volumes superior to those previously reported. Lamarre et al. described a mean clearance of 0.103 L/h/kg, in a population of 98 general pediatric patients, with a mean distribution volume of 0.43 L/kg.16 The present values were, respectively, 0.18 L/h/kg and 1.02 L/kg.
The proportion of patients in which vancomycin therapeutic levels were obtained with usual doses was similar to that reported in adults by Lepe et al. (50%).17
Vancomycin induces bacterial death in a time-dependent manner, with a moderate post-antibiotic effect. The necessary dose to achieve recommended serum levels can be extremely high, with increasing possibility of nephrotoxicity.18 In an effort to establish a mathematical model that could quickly suggest the ideal dose for adults with malignant hematologic diseases, Buelga et al. analyzed data from 215 patients with 1,004 serum dosages of vancomycin. It was observed that the patient weight was strongly correlated with distribution volume, while creatinine clearance and the diagnosis of acute mieloblastic leukemia influence vancomycin clearance. The authors proposed the following general model to estimate vancomycin clearance (Vclear): Vclear=1.08×estimated creatinine clearance. The equation for distribution volume (DV) was: DV=0.98×weight (kg). This model was able to predict vancomycin clearance with a precision of ±20% in 33% of cases.19
In the present sample, the parameter that showed a better correlation with vancomycin clearance was serum creatinine, and the model derived from the fitting line was able to predict the clearance in 41% of cases (similar to the 33% found by Buelga et al.19), with a tendency to overestimate the lower values. Estimating the creatinine clearance with the Schwartz's formula showed no utility. It must be observed that Buelga's model presented, according to the authors, at least two times more precision than the normally utilized nomograms. Pharmacokinetical Bayesian analysis can allow individual adjustments and could be more useful than the pure mathematical models. From a single serum measure of creatinine and vancomycin at steady-state (at any moment after the fourth dose and after the drug distribution phase, one to two hours after the end of infusion), it is theoretically possible to estimate the ideal dose required to achieve the intended trough levels.17 This approach could avoid the inconvenience of raising the dose by trial and error and reduce the costs of multiple measures and the risk of nephrotoxic supratherapeutic levels, but prospective studies are lacking. Because of its complexity, Bayesian analysis demands specific softwares to be accessible to clinicians. Some commercial options are available, with moderate to high cost, and the JPKD software is free.
The highest doses used were relatively well tolerated, with only 6.4% of patients having significant elevations of serum creatinine.
ConclusionThis study shows that higher than usual vancomycin doses may be required to treat staphylococcal infections in children with oncologic/hematologic diseases. Since the best known predictor of efficacy is the AUC/MIC ratio, serum trough levels must be analyzed in conjunction with MICs of prevalent Staphylococci and pharmacokinetic tools such as Bayesian analysis.
Conflict of interestAll authors declare to have no conflict of interest.
The authors like to thank Yung-jin Lee, PhD, and the JPKD team, College of Pharmacy, Kaoshiung Medical University, Taiwan.